Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Full article: Real-world impact and effectiveness of MenACWY-TT
Full article: Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study

PDF) A phase 2b/3b MenACWY-TT study of long-term antibody persistence after primary vaccination and immunogenicity and safety of a booster dose in individuals aged 11 through 55 years

Full article: Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age

Systematic literature review of the impact and effectiveness of monovalent meningococcal C conjugated vaccines when used in routine immunization programs, BMC Public Health
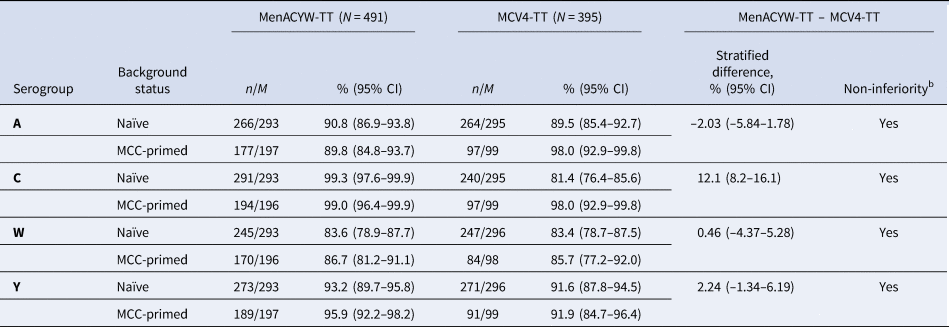
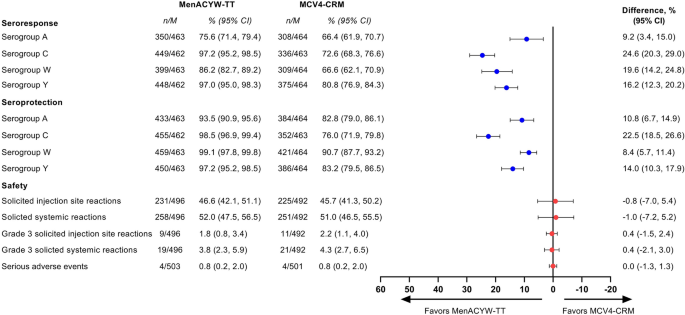
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
PDF) Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Microorganisms, Free Full-Text

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health

hSBA vaccine seroresponse a after a booster dose of MenACYW-TT at (a)