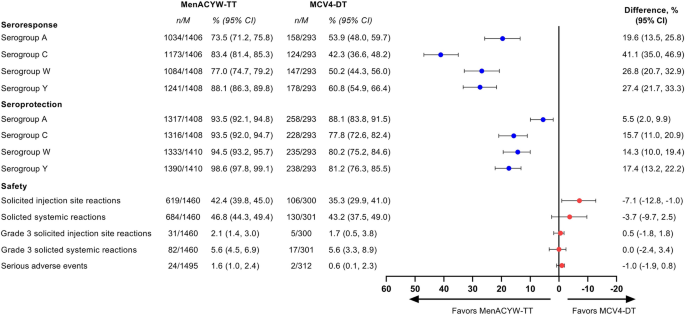
Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy

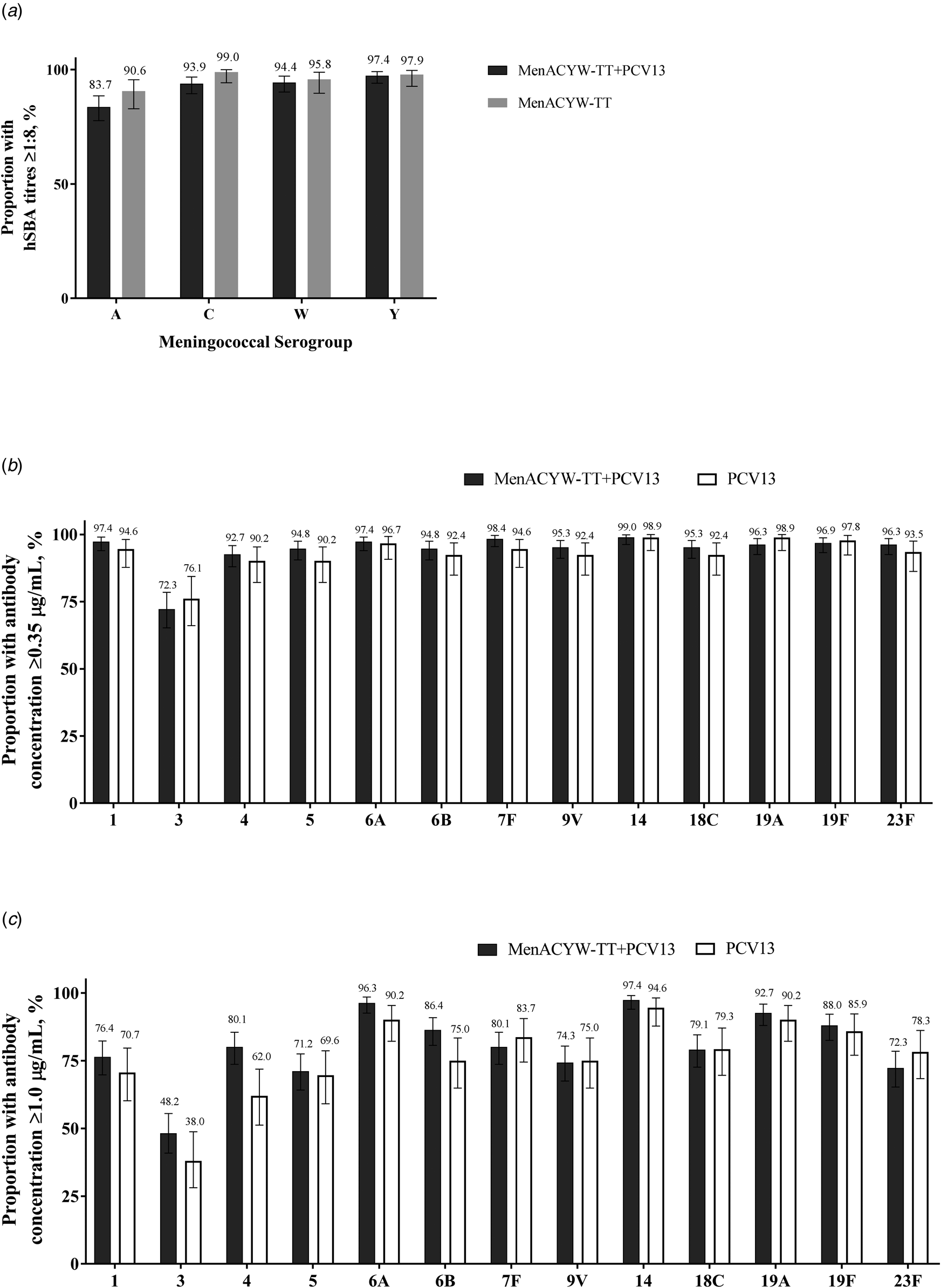
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study, Epidemiology & Infection
Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate v
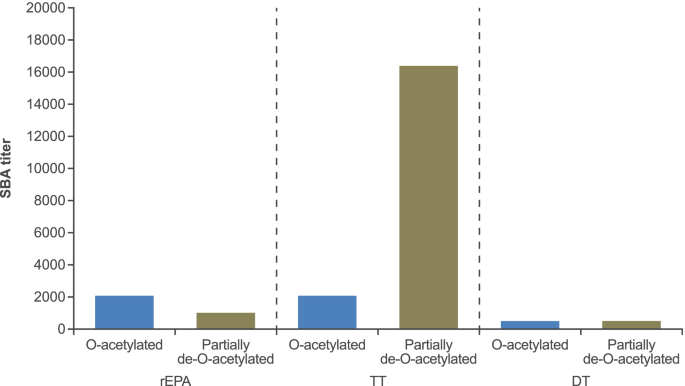
Preclinical development of the quadrivalent meningococcal (ACYW) tetanus toxoid conjugate vaccine, MenQuadfi®
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Full article: Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study
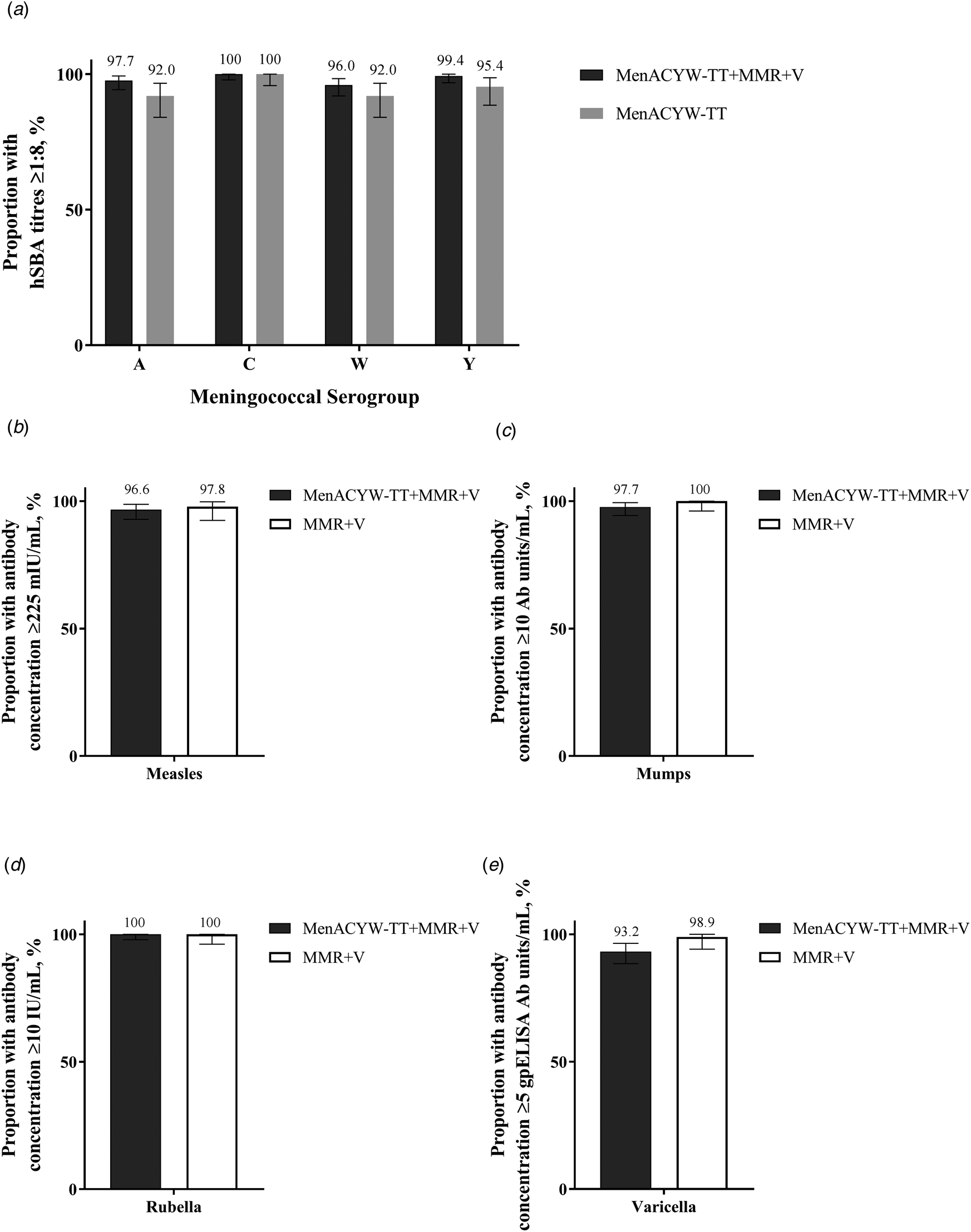
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study, Epidemiology & Infection

Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia

Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia

Full article: Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study

Preclinical development of the quadrivalent meningococcal (ACYW) tetanus toxoid conjugate vaccine, MenQuadfi®

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label




.JPEG)

