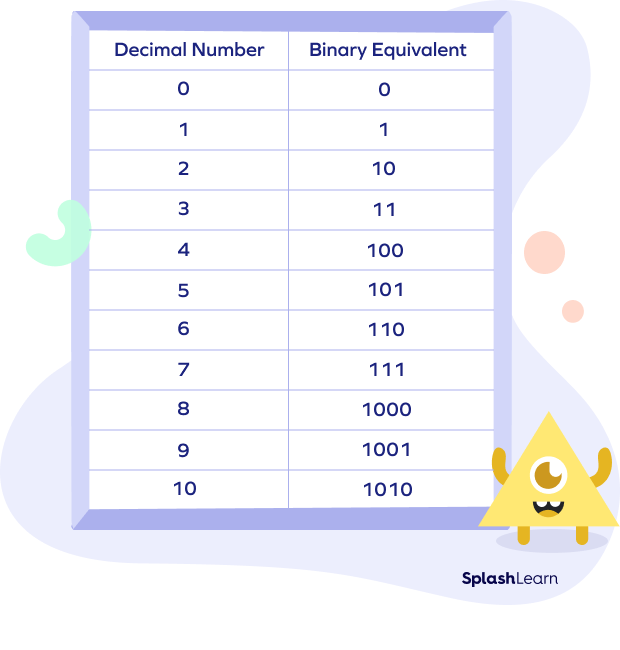
Classification of Bases - on Basis of Solutibility, Ionising Capacity

Bases can be categorised based on different properties:On the Basis of Solubility:Bases can be classified asSolubleandInsoluble.Soluble Bases: These are bases which are dissolvable in water. These are also calledAlkalis. For Example : Sodium Hydroxide (NaOH), Potassium Hydroxide (KOH)Insoluble Bases

UNIT II: Acid, Base and Buffer

Degree of Ionization - an overview
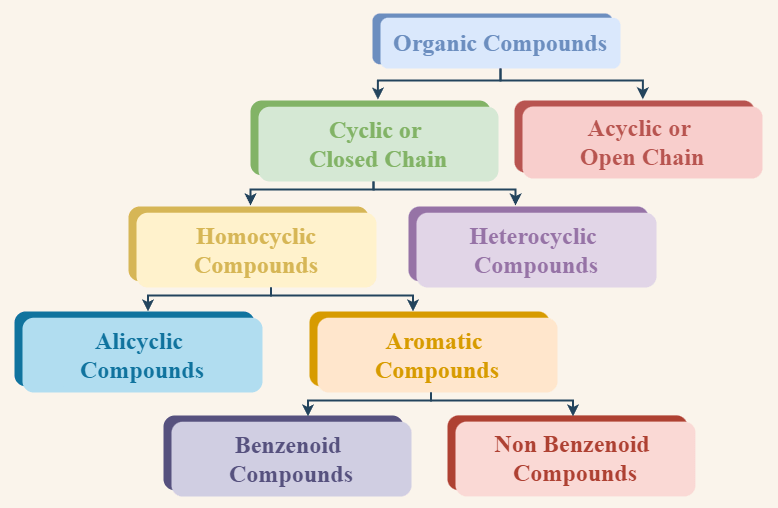
Classification of Organic Compounds with Structure and Examples
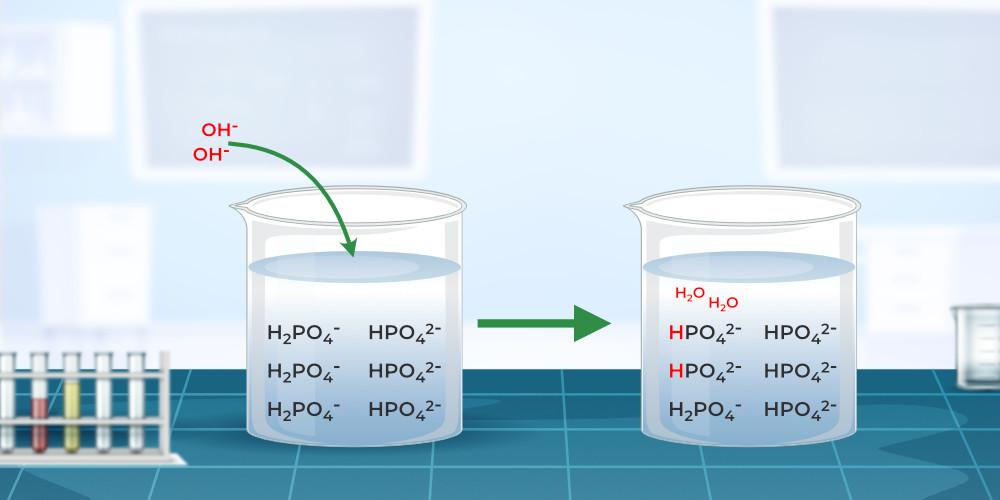
Buffer Solution - Definition, Types, Formula, Examples, and FAQs

Acid & Base Properties of Water, Overview & pH Measurement - Video & Lesson Transcript

Synergy between Ionic Capacity and Intrinsic Porosity in Imidazolium-Based Cationic Organic Polymers and Its Effect on Anionic Dye Adsorption

Classification of Bases - on Basis of Solutibility, Ionising Capacity

Base (chemistry) - Wikipedia

Interplay of Acid–Base Ratio and Recycling on the Pretreatment Performance of the Protic Ionic Liquid Monoethanolammonium Acetate

PHARMACEUTICAL ANALYSIS I - ACID BASE TITRATIONS
PPT - Acids and Bases PowerPoint Presentation, free download - ID:3842068

Empirical Conversion of pKa Values between Different Solvents and Interpretation of the Parameters: Application to Water, Acetonitrile, Dimethyl Sulfoxide, and Methanol

Difference Between Acid and Base: Table, Properties, Uses



/cdn.vox-cdn.com/uploads/chorus_image/image/72331329/1245874313.0.jpg)