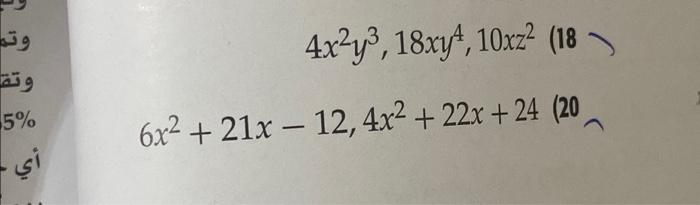c) 20,000 200000 13. An element, X, have three isotopes 22X. The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be ((a) 9% (b) 8% (

Click here:point_up_2:to get an answer to your question :writing_hand:c 20000let 20000013 an element x have three isotopes22x the percentage abundance ofits average atomic
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The
Element X has two isotopes of 16 and 18. Its relative atomic mass

SOLVED: An unknown element X has the following isotopes: ¹⠰X

Isotopes AND % Abundance - SSC Chemistry
The three stable isotopes of neon: 20,10 Ne 21,10Ne and 22,10 Ne

Solved Consider an element Z that has two naturally
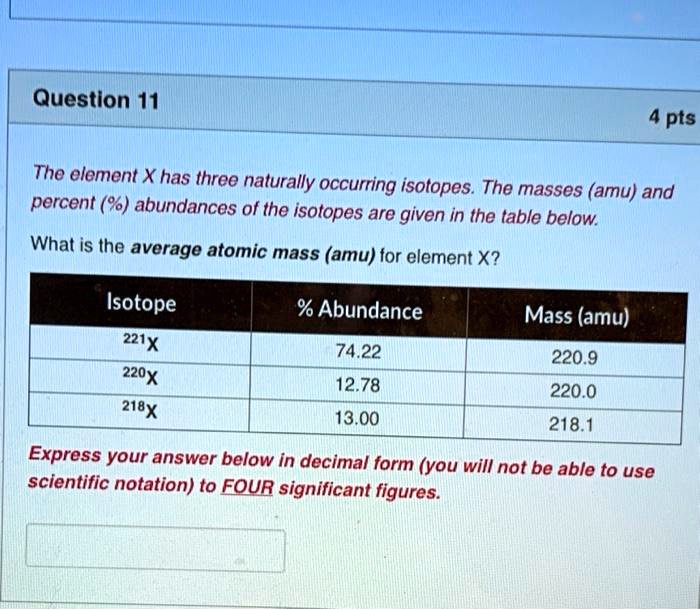
SOLVED: The element X has three naturally occurring isotopes. The

SOLVED: The element X has three naturally occurring isotopes. The
Element Z has 2 isotopes. A sample of element Z contains 19% Z-234

An element exist in three isotopic form 40x, 41X and 42x