If 900J//g of heat is exchanged at boiling point of water then water


59. If 900 J/g of heat is exchanged boiling point of water, then what is increase in entropy? (1) 900 K/mol (2) 87.2 J/mol (3) 43.4 J/mol • (4) Zero Amb fthe followinerentianale..

Experimental values for the thermal diffusivity of 304 stainless steel.
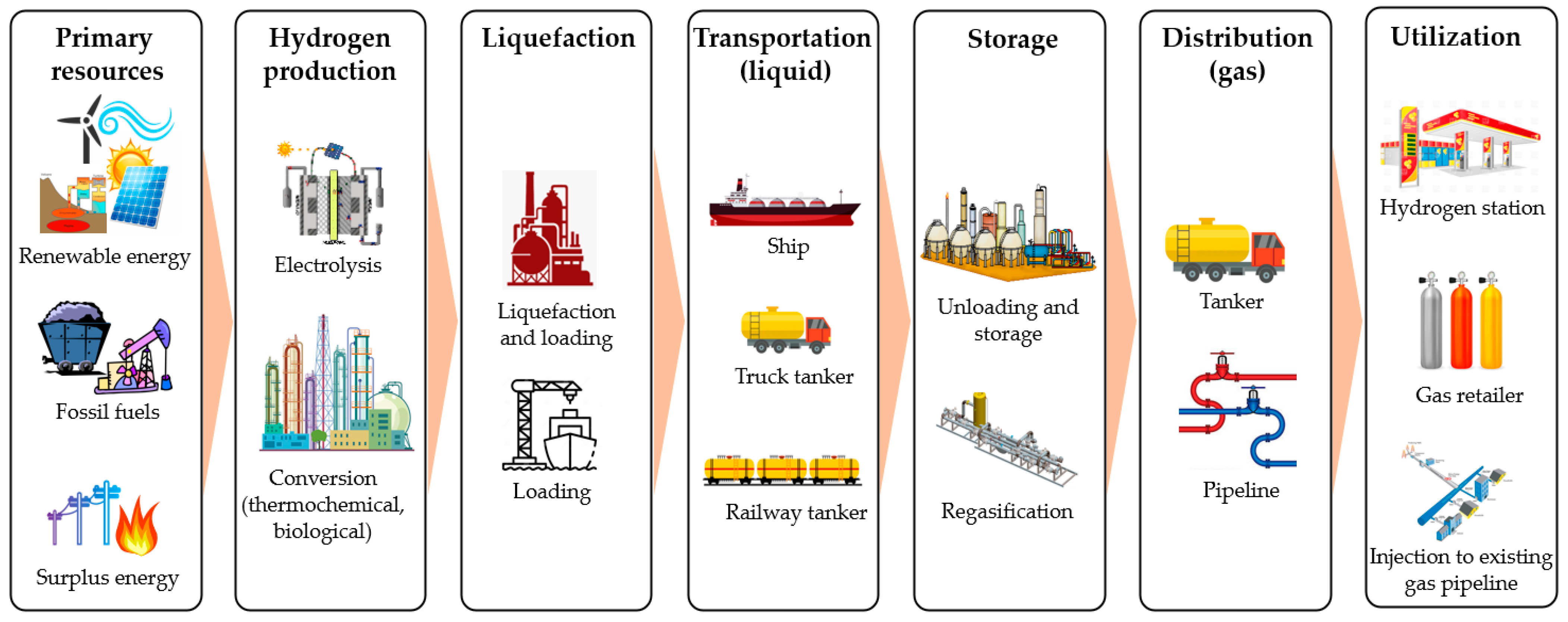
Energies, Free Full-Text
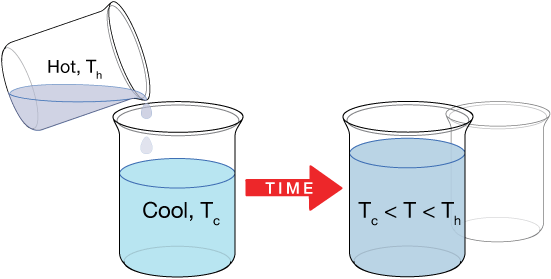
Thermal equilibrium

Integration (Chapter 6) - Water Quality Impacts of the Energy-Water Nexus
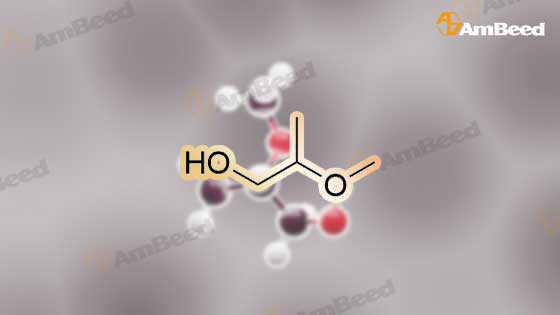
1589-47-5, 2-Methoxypropan-1-ol

Impact of instantaneous solar irradiance on refrigeration characteristics of household PCM storage air conditioning directly driven by distributed photovoltaic energy - Xu - 2022 - Energy Science & Engineering - Wiley Online Library

A Thermodynamics Model for the Assessment and Optimisation of Onboard Natural Gas Reforming and Carbon Capture

Wolfram: Eigenschaften & Verwendung

ene 64) If 900 J gf of heat is exchanged boling point of water then increase in entropy is 1) 43.4 J K mot 2) 87.2 J K molt 3) 900 J K mot 4) zero

Given the phase diagram of water below, what are the values of its critical temperature and critical pressure? Also, at 0 degrees C, what kind of phase changes would occur as the

If 900 J/g of heat is exchanged at boiling point of water, then what is increase - NEETLab
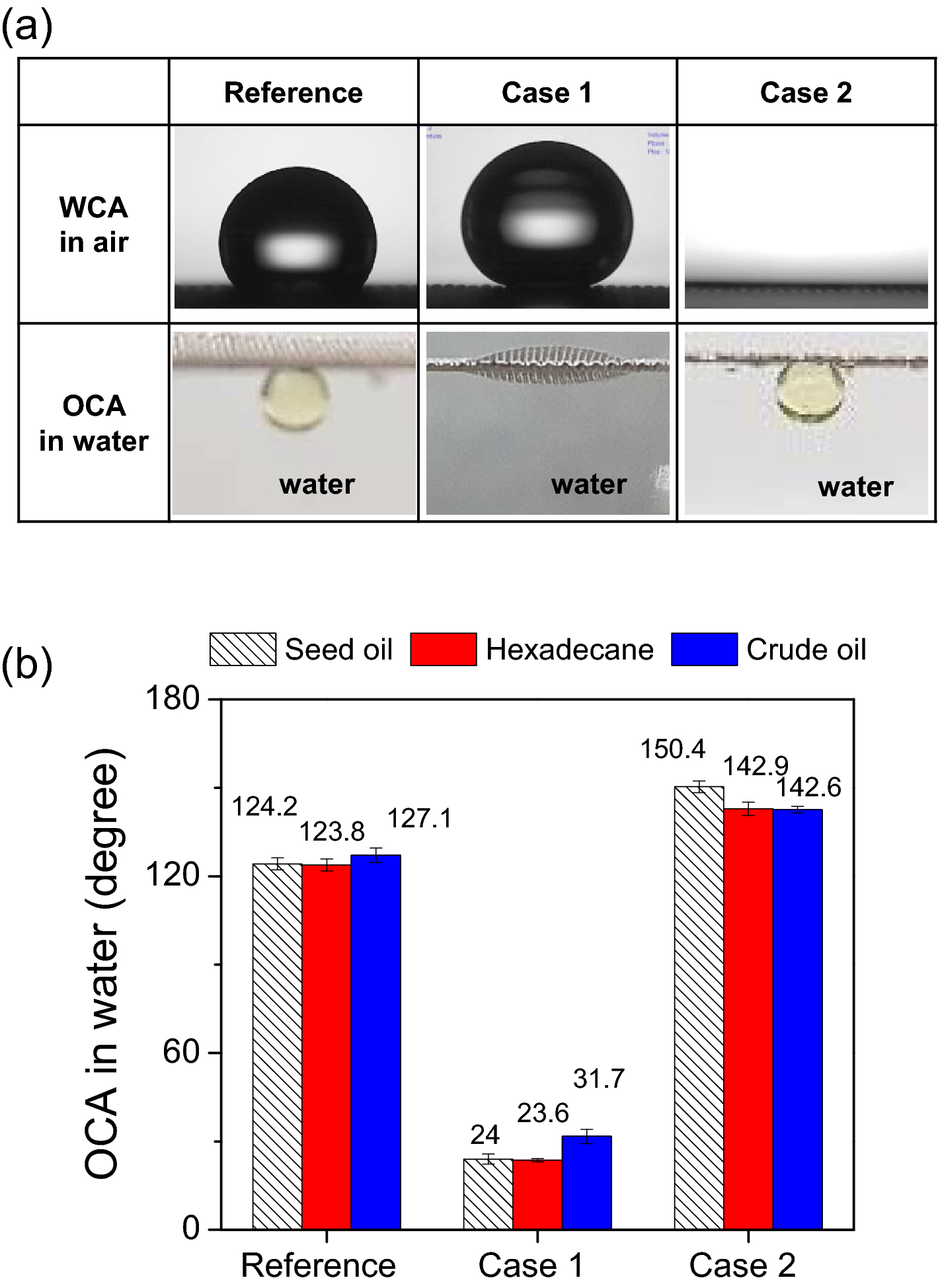
Simultaneous, efficient and continuous oil–water separation via antagonistically functionalized membranes prepared by atmospheric-pressure cold plasma









