Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in

Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study - The Lancet Infectious Diseases

Vaccines, Free Full-Text

Neutralizing antibodies induced by homologous and heterologous boosters in CoronaVac vaccines in Chile - Clinical Microbiology and Infection

Full article: Evidence synthesis and pooled analysis of vaccine effectiveness for COVID-19 mRNA vaccine BNT162b2 as a heterologous booster after inactivated SARS-CoV-2 virus vaccines

A comparative characterization of SARS-CoV-2-specific T cells induced by mRNA or inactive virus COVID-19 vaccines - ScienceDirect

Effectiveness of BNT162b2 and CoronaVac vaccines against omicron in children aged 5 to 11 years

NEJM — Covid-19 Vaccine Resources

Booster dose of BNT162b2 after two doses of CoronaVac improves neutralization of SARS-CoV-2 Omicron variant
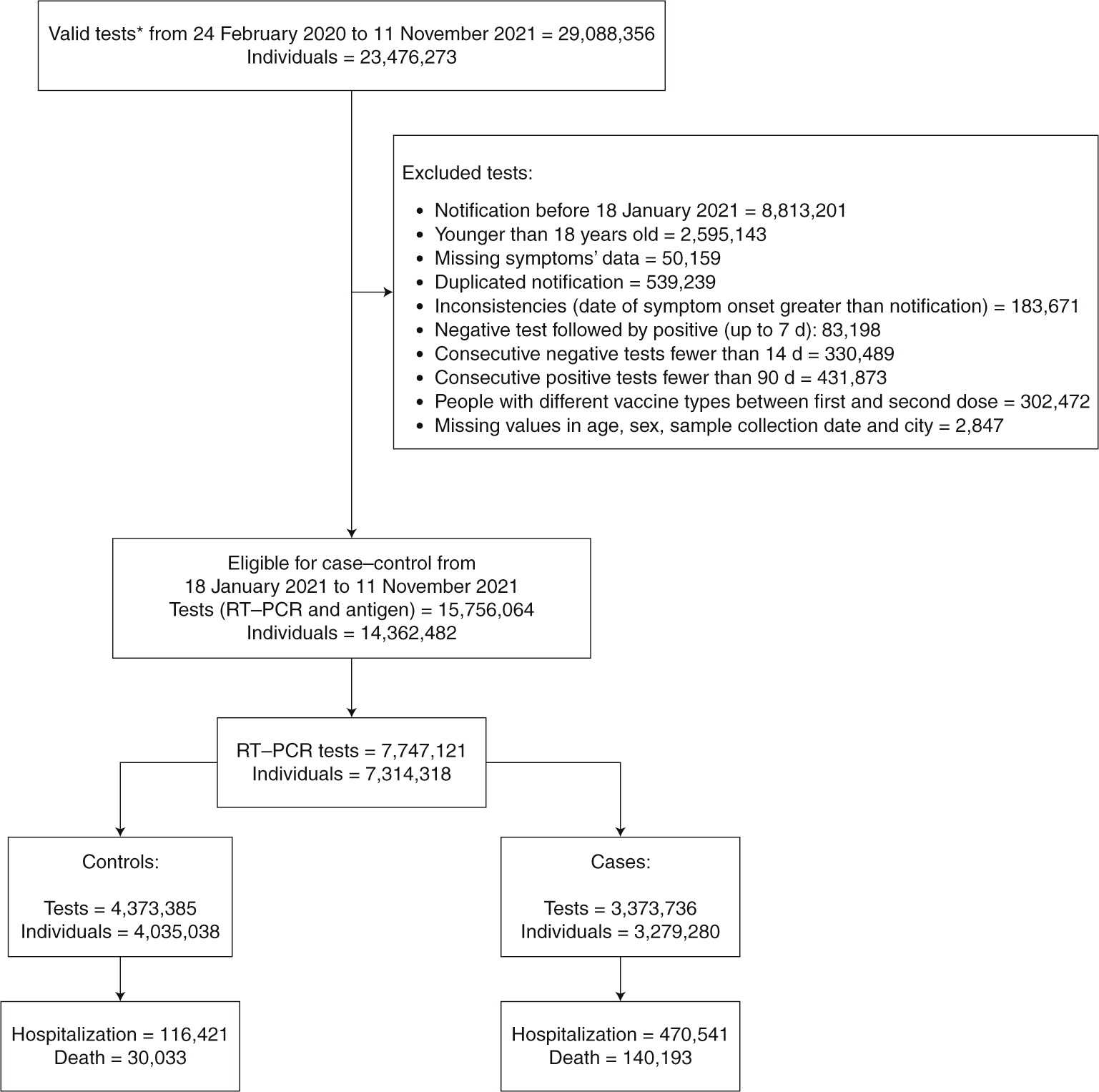
Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland

Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac and ChAdOx1 and BNT162b2 plus heterologous BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults
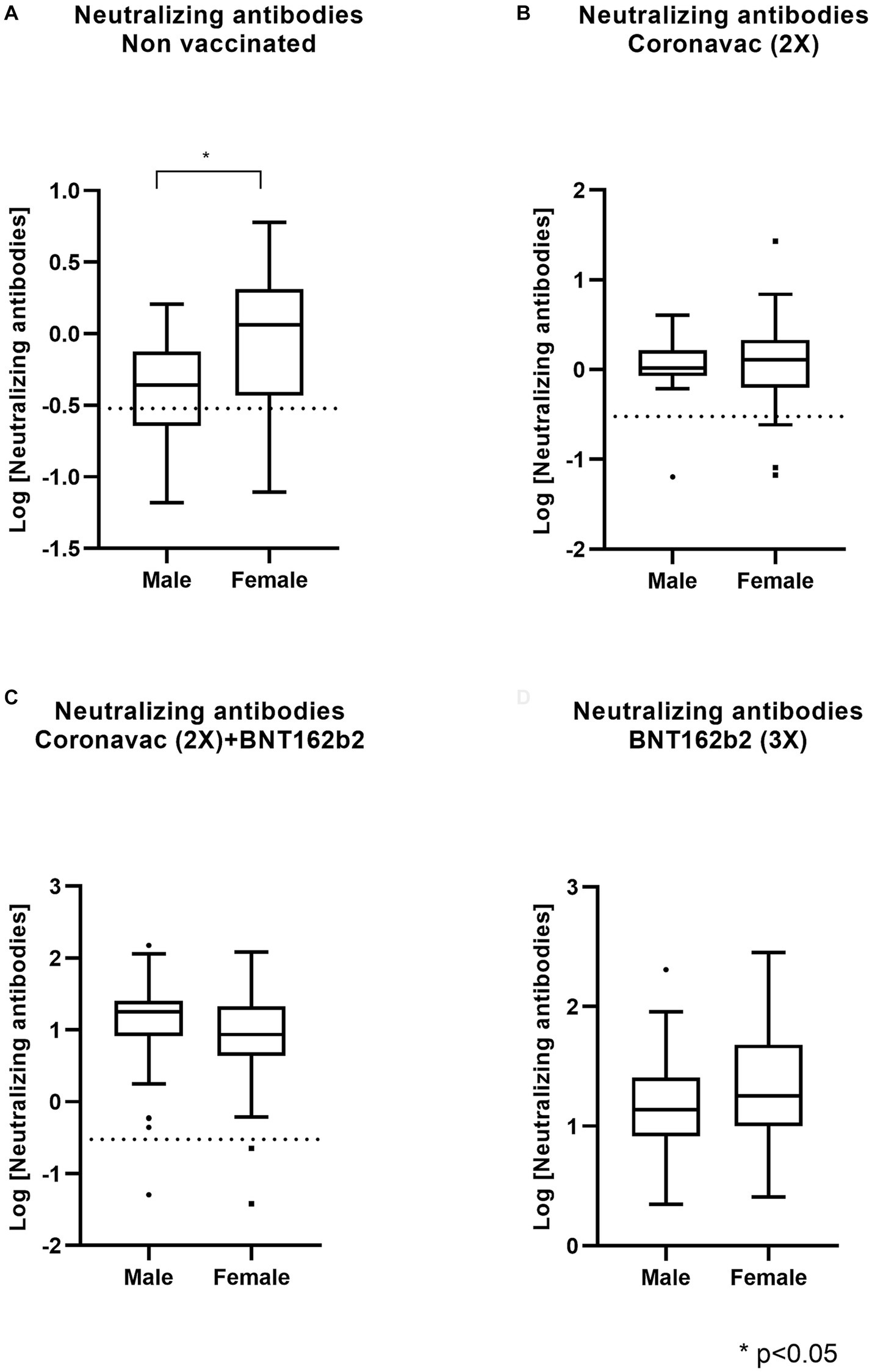
Frontiers Humoral immunity against SARS-CoV-2 evoked by heterologous vaccination groups using the CoronaVac (Sinovac) and BNT162b2 (Pfizer/BioNTech) vaccines in Chile