What Is Regulatory Dossier and What Does It Contain? - The


CTD Dossier Preparation. Sr.Manager-Regulatory Affairs - PDF Free Download

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy Methods & Clinical Development

Regulatory dossier preparation and submission as per CTD format
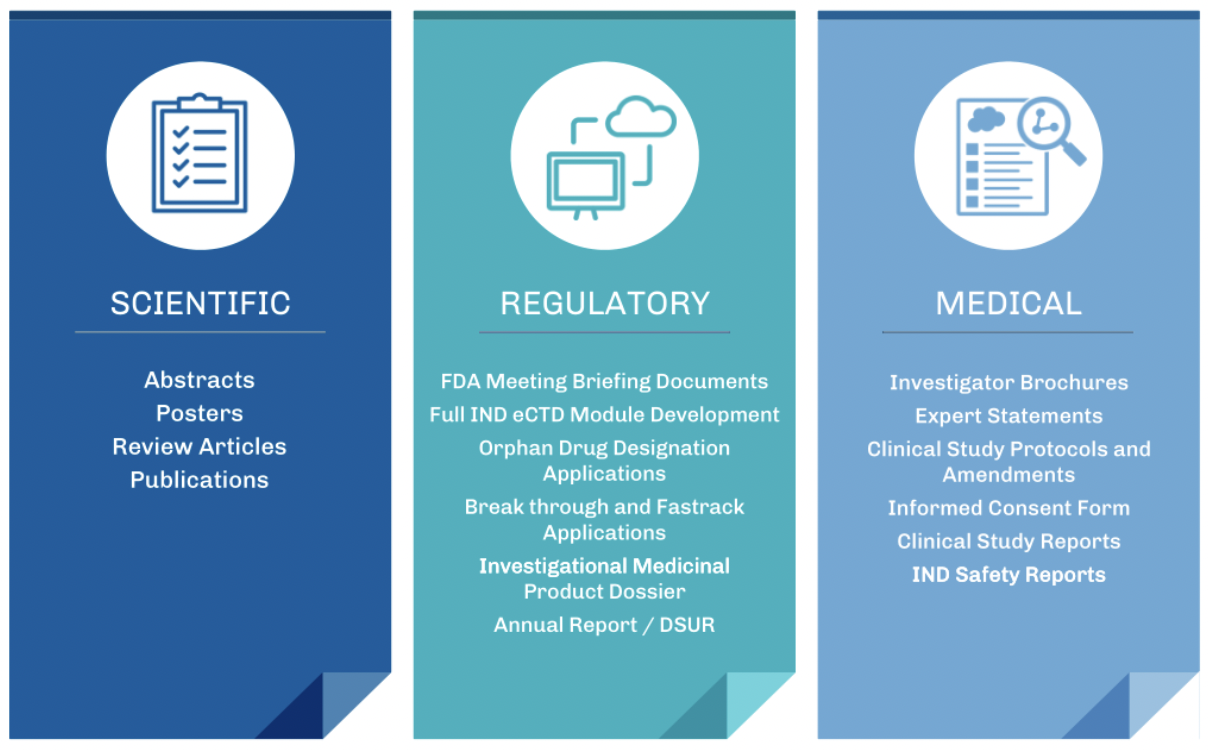
LP – Regulatory, Scientific & Medical Writing - TD2 Precision Oncology
Dynamic Dossier in the Cloud Center for Biomedical System Design
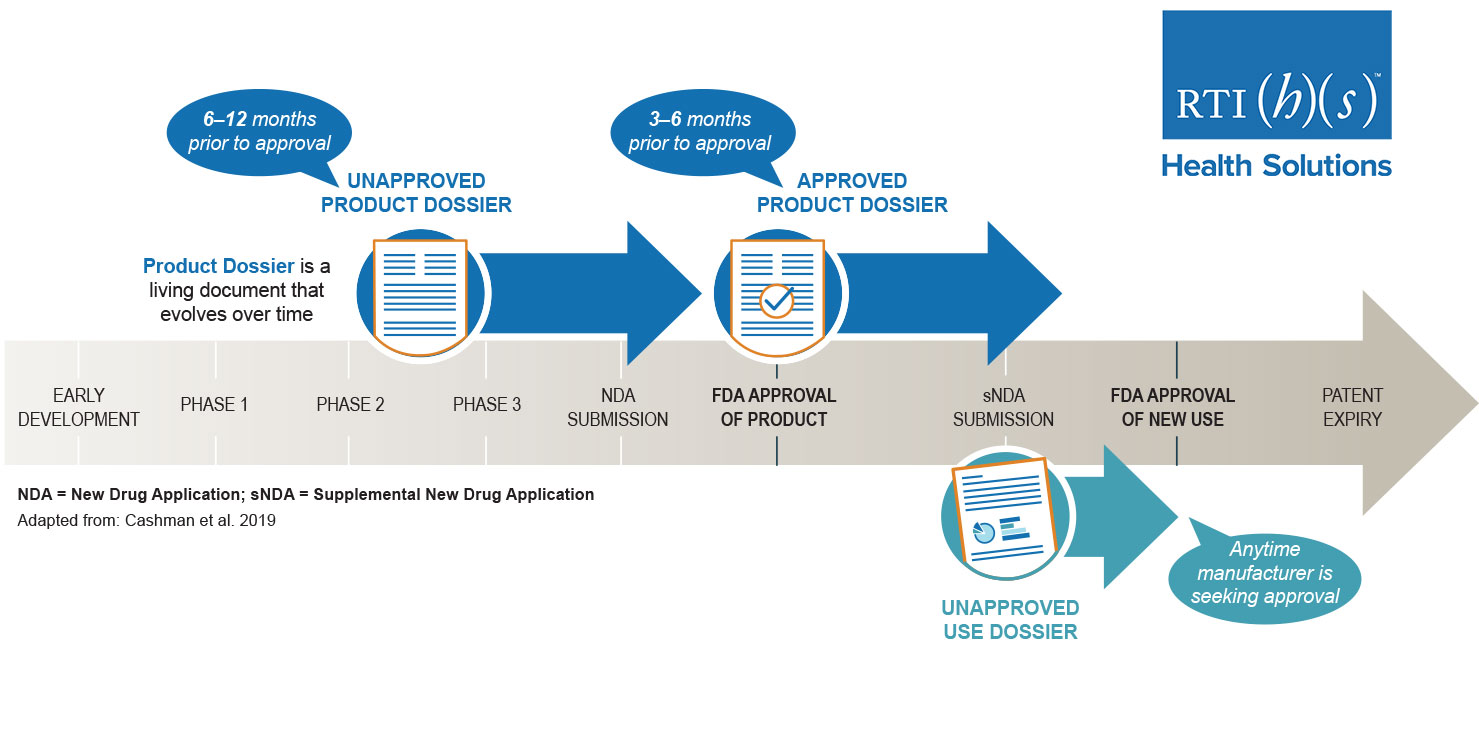
PIE and Post-Approval AMCP Dossiers

PDF) Regulatory requirements for Dossier submission in African Countries (Kenya, Uganda, and Tanzania) - A Review
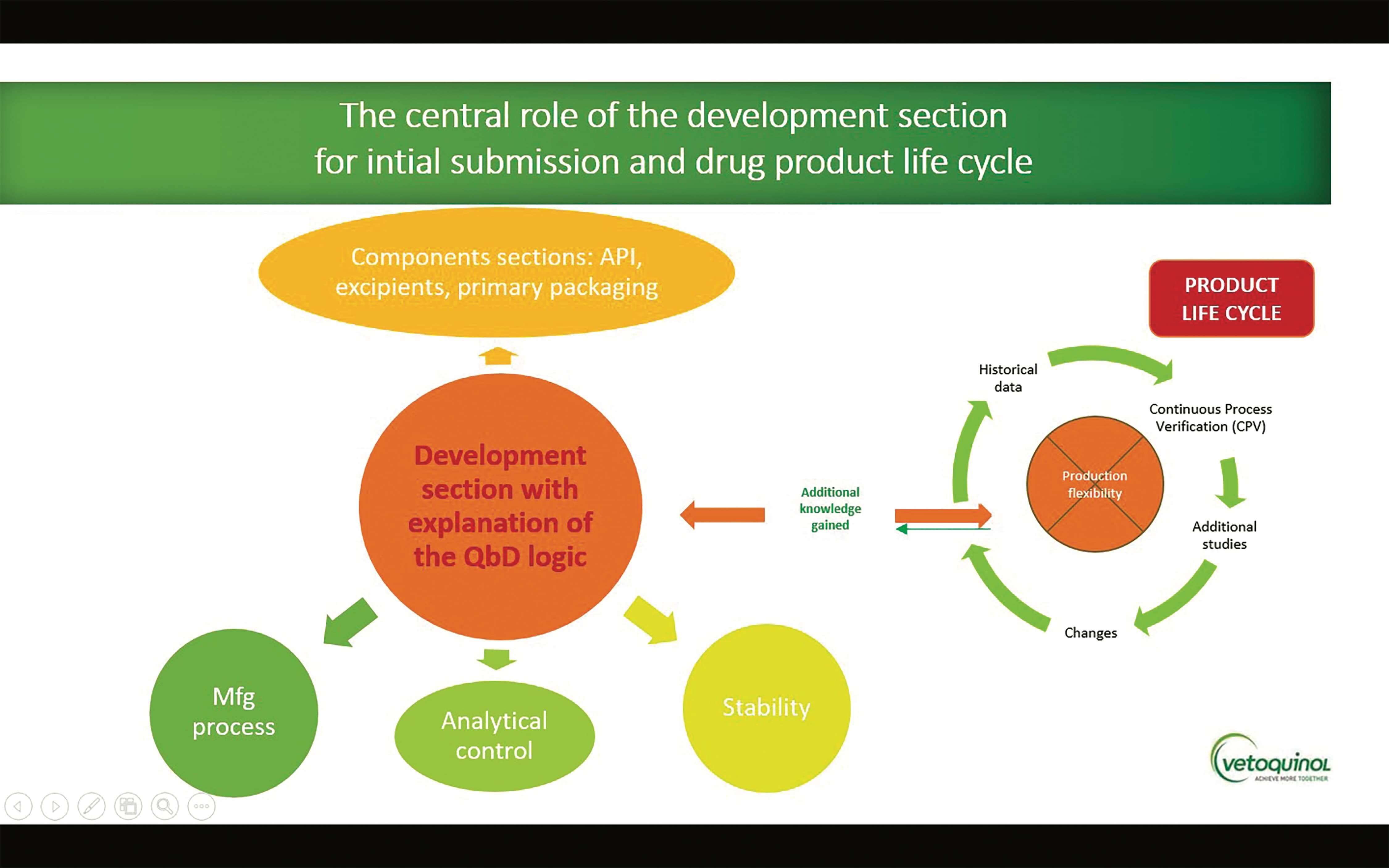
First steps towards ICH Q12: Leveraging process understanding & development data to define process Established Conditions - A3P - Pharmaceutical & Biotechnology Industry

Unlocking The Value Of Dossiers with eCTD Viewing Technology

What Is Regulatory Dossier and What Does It Contain? - The Kolabtree Blog

Indian Regulatory Dossier preparation - Things to remember

Industry perspectives on implementation of Quality Overall Summary-Product Dossier (QOS-PD) and Quality Information Summary (QIS) for innovative medicinal products - IFPMA
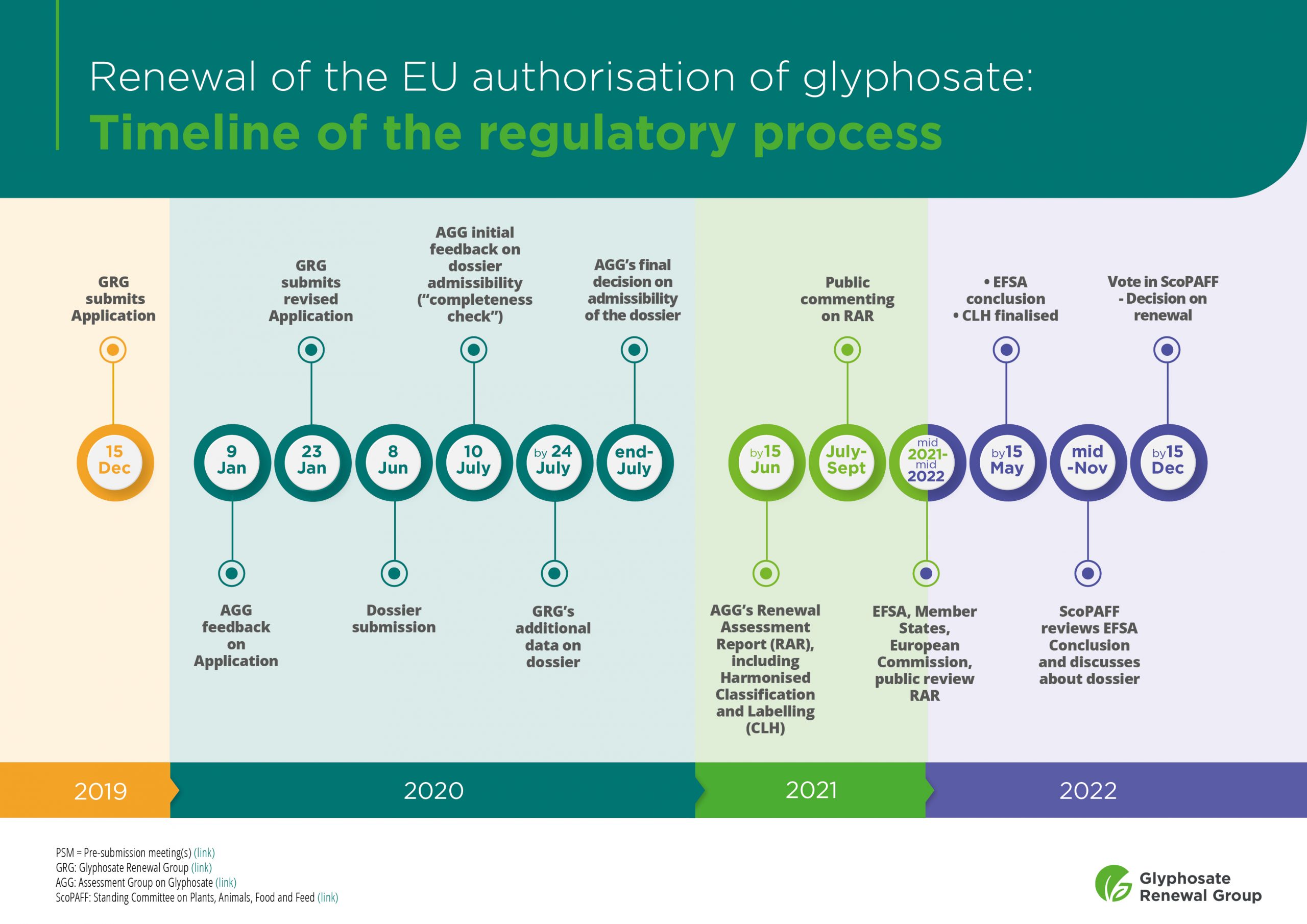
The new glyphosate dossier is admissible - Glyphosate Renewal Group








