Difference between Strong and Weak Base - with Examples [in Table]
![Difference between Strong and Weak Base - with Examples [in Table]](https://d1avenlh0i1xmr.cloudfront.net/0e323ff3-079c-4d5e-b21f-0f4c25082521/differences-between-strong-and-weak-bases-01.jpg)
Strong BaseWeak BaseThey get completely ionized (split up into ions) in water and produce large amounts of hydroxide ions.These only get partially ionized (split up into ions) in water and produce less amount of hydroxide ions.pH value is close to 14 but smaller than it.pH value is closer to 7 but
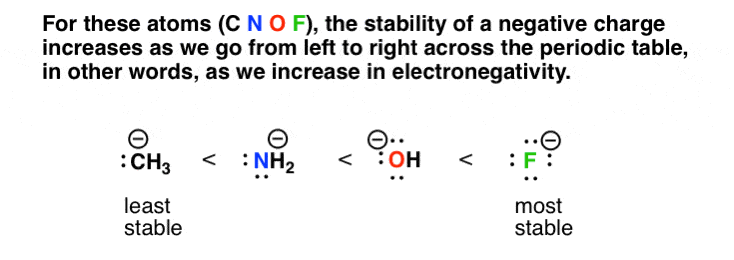
Basicity Is Another Word For Stability Of A Lone Pair Of Electrons
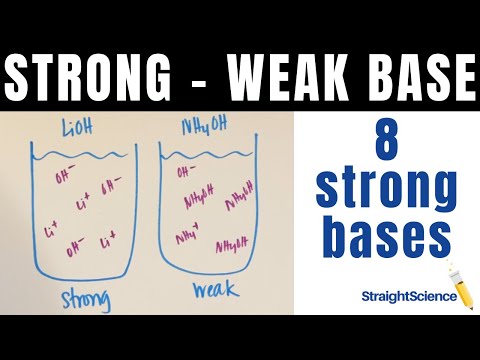
Strong vs. Weak Bases - What's the difference? How do they dissociate? The 8 Strong Bases
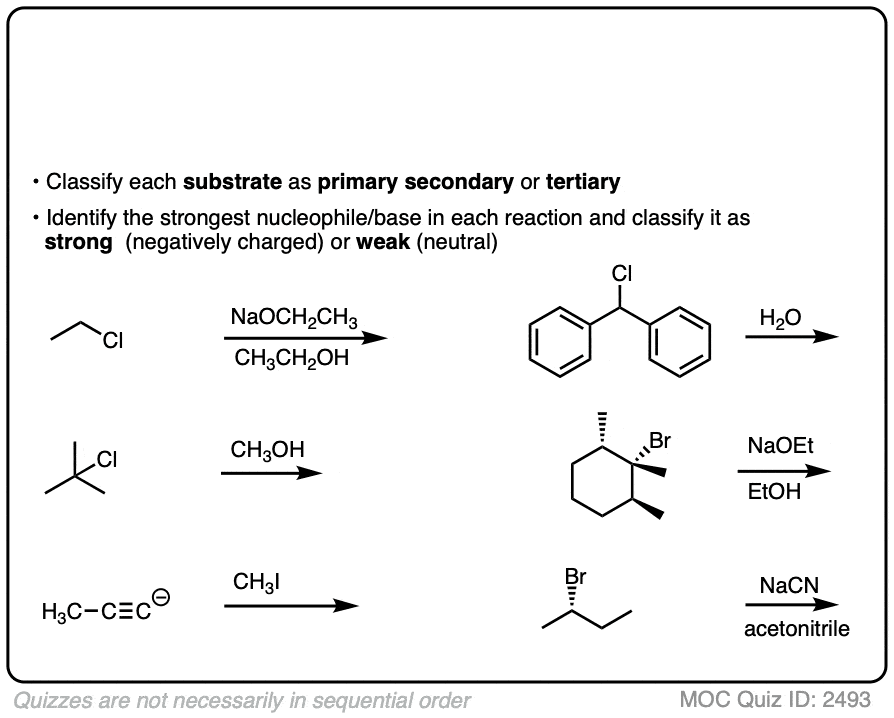
Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
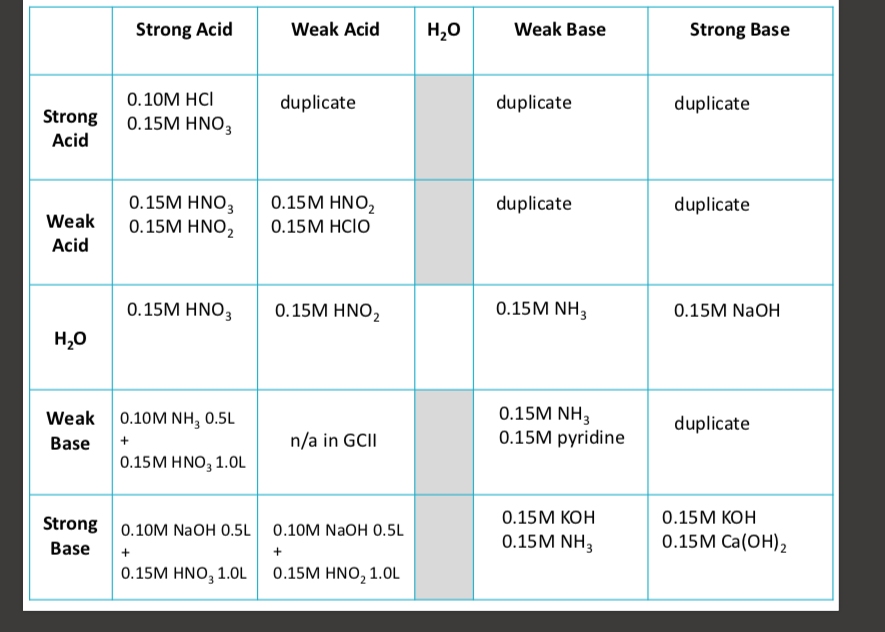
write a process of finding pH (with

SN1 SN2 E1 E2 - How to choose the coorect mechanism
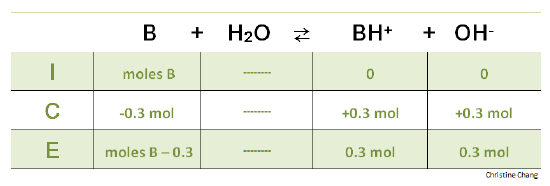
Titration of a Weak Base with a Strong Acid - Chemistry LibreTexts
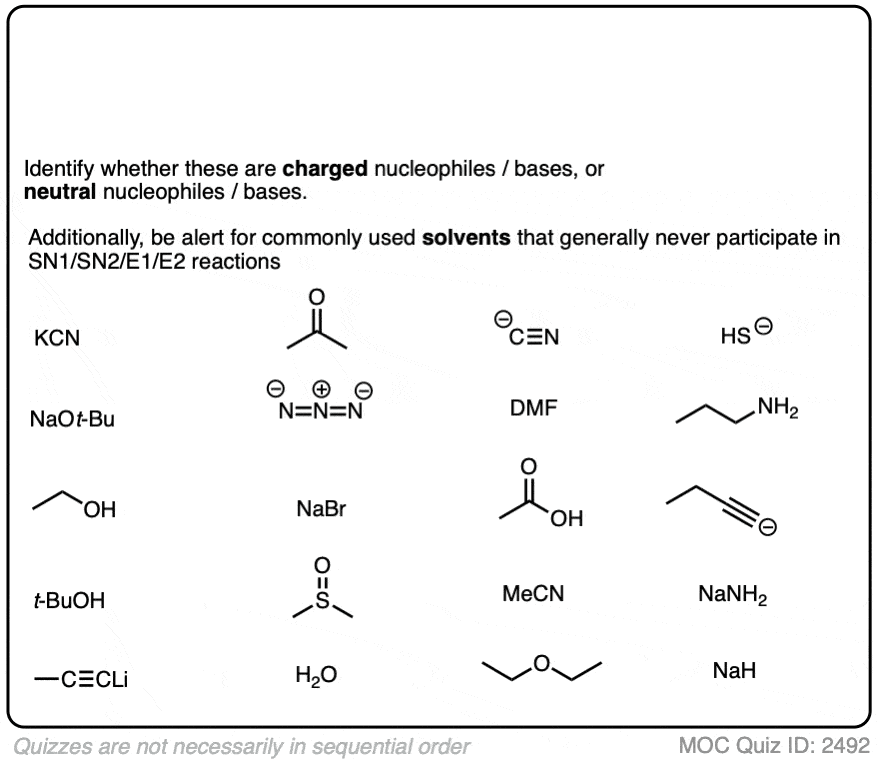
Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
Which indicator is used in a strong acid versus a strong base solution? - Quora

10.3 Strong and Weak Bases LO: I understand the difference between strong and weak bases. - ppt download
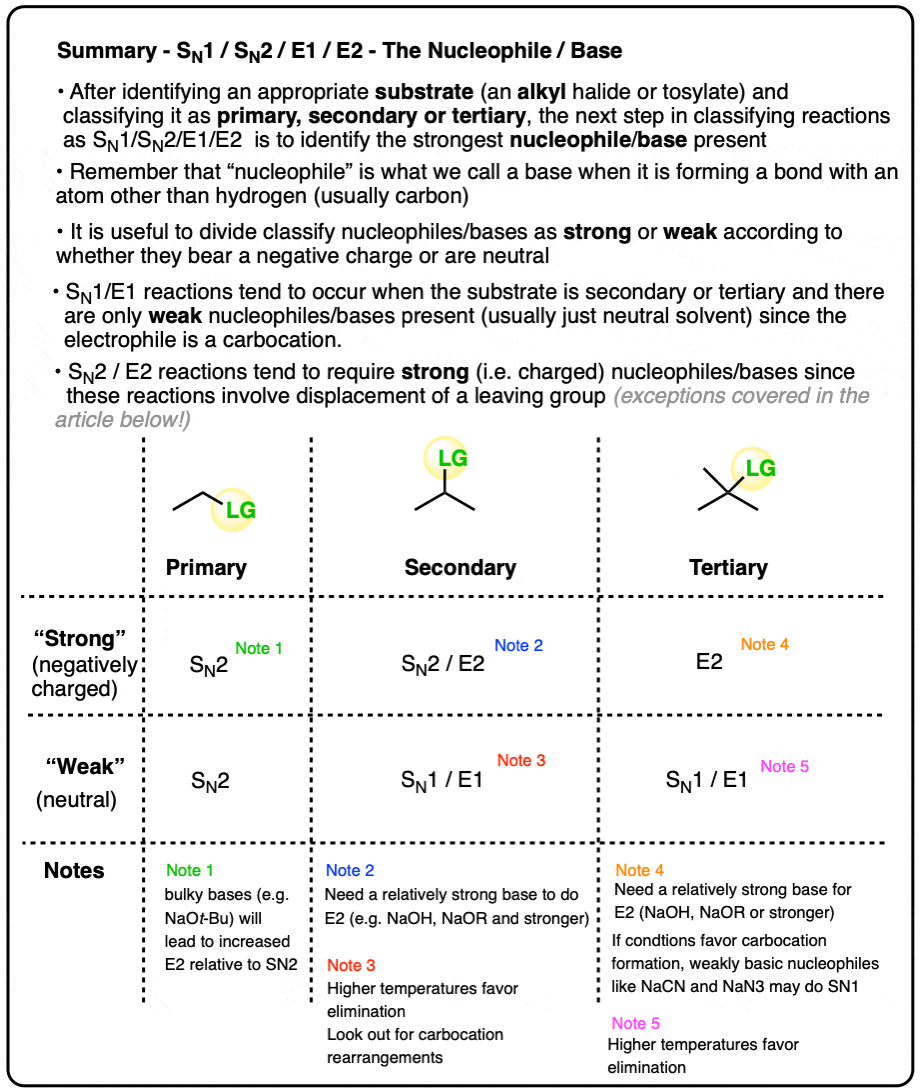
Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base

Examples of Weak Base (5 Examples with Images) - Teachoo Chemistry

What is the difference between a weak base and a strong base? - Quora
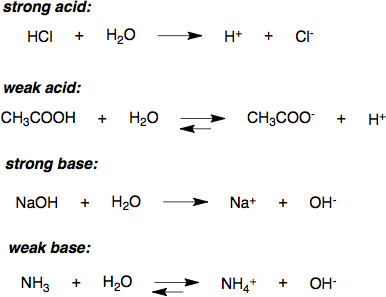
Strength of Acids – Introductory Chemistry





:max_bytes(150000):strip_icc()/Term-Definitions_Base-year-blue-ff64d3c5ba93495d97c209b358d51423.jpg)


