Why Is Water a Polar Molecule?
:max_bytes(150000):strip_icc()/GettyImages-1041588324-5c3cf475c9e77c0001d63bca-5c3f692fc9e77c0001d9a10f.jpg)
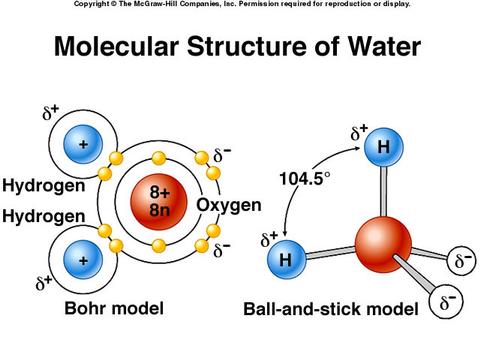
Water is a polar molecule because the electrons are unevenly distributed. Since the molecule is polar, water is a polar solvent, also.

Is H2O(Water) polar or nonpolar?
Water A Polar Molecule on Vimeo

Polar Molecule, Definition, Characteristics & Examples - Lesson

Properties of Water Polar molecule Hydrogen bonding Surface tension Cohesion and adhesion Universal solvent. - ppt download

What is meant by polar water molecules? - Quora
Art Quiz

Polar and Non-Polar Molecules

Why is water (H2O) a polar molecule?

Amna's Personal Site
Tu pourrais aussi aimer









