Understanding The FDA's Current Focus On Risk Evaluation And

lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>

The FDA food code for 2023 and it's significance for businesses

FDA-cleared artificial intelligence and machine learning-based

DCE, discrete choice experiment; FDA, US Food and Drug
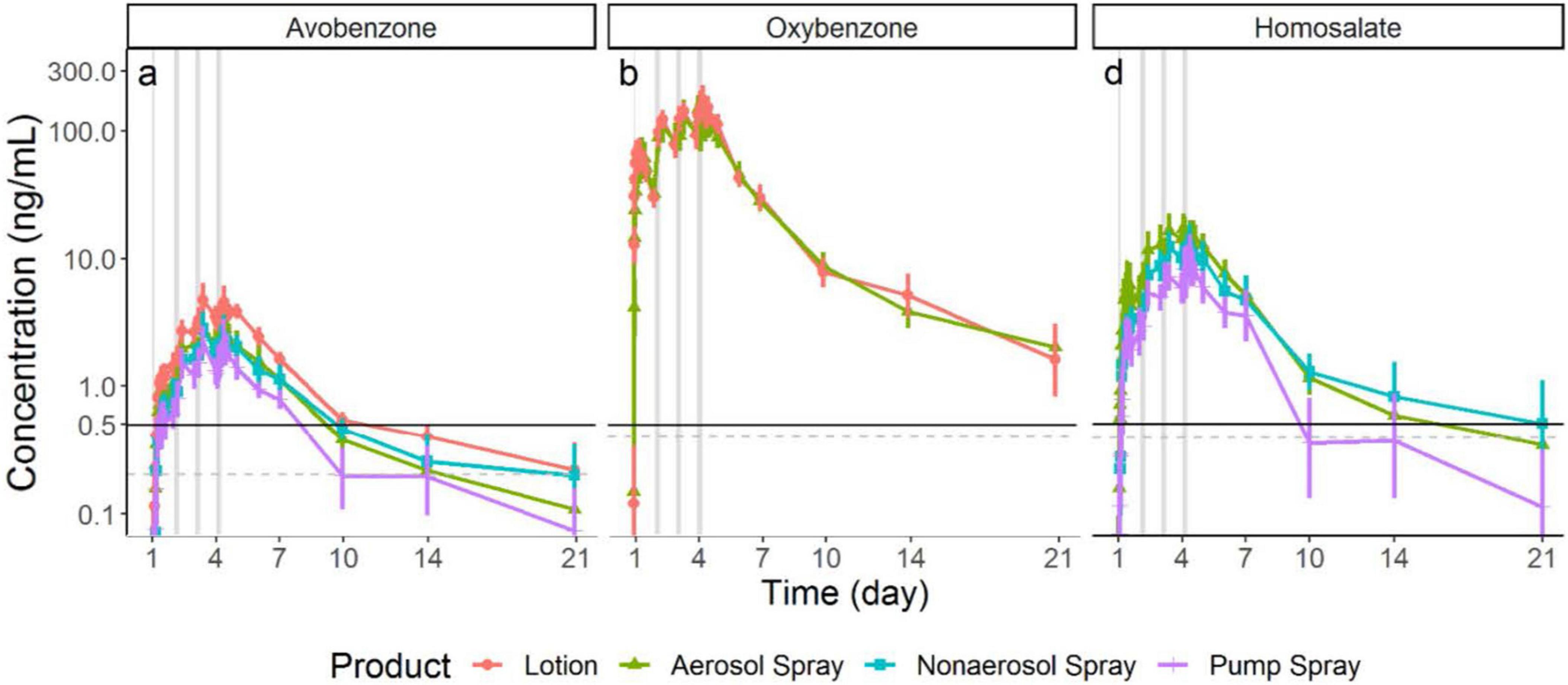
Frontiers New science, drug regulation, and emergent public
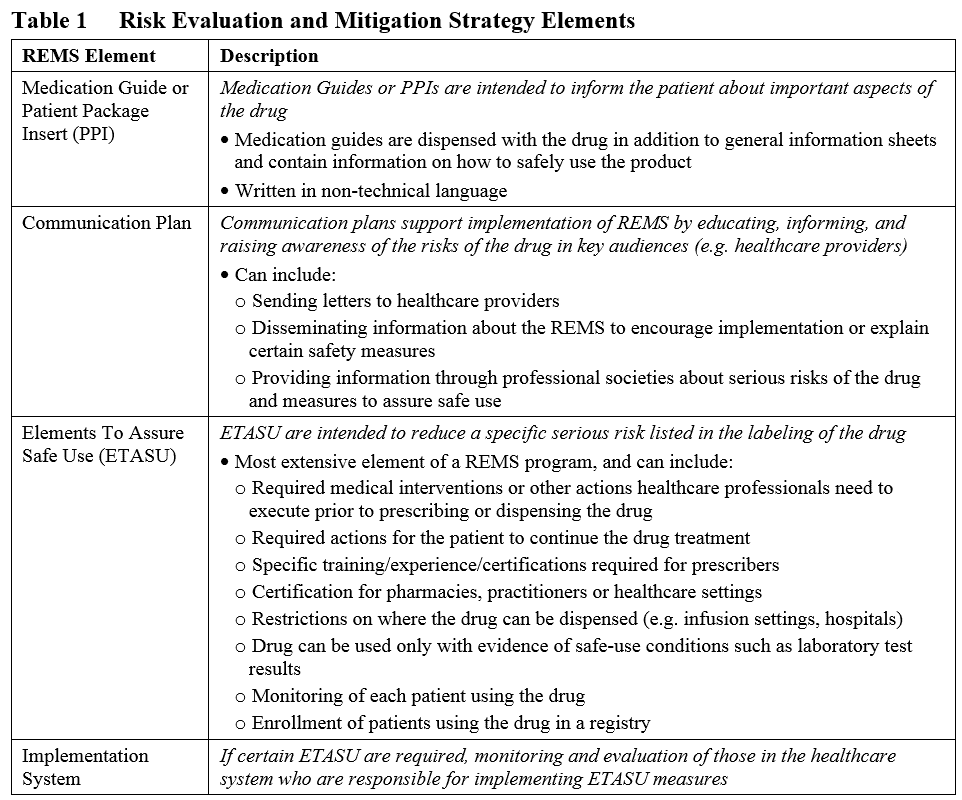
Risk Evaluation And Mitigation Strategies (REMS) Basics
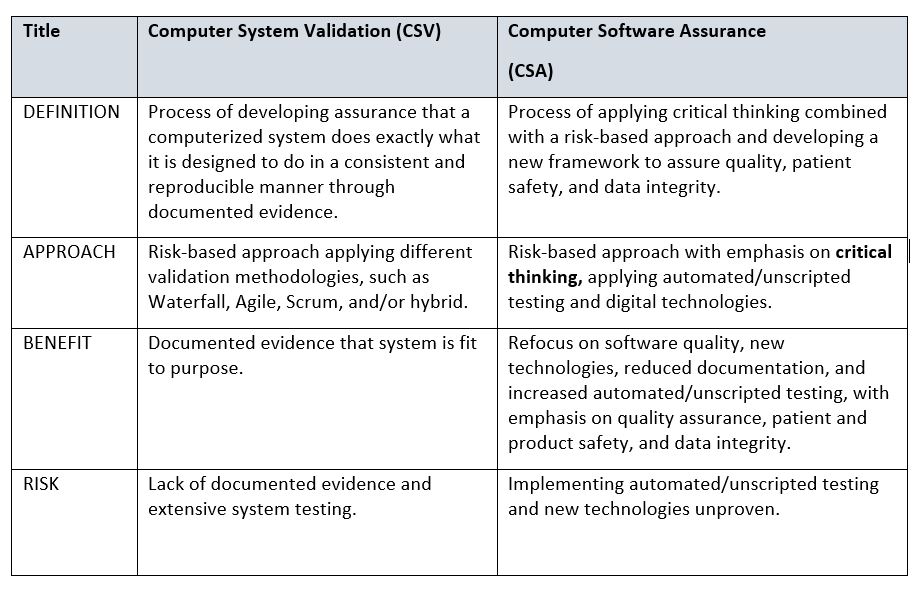
Are You Ready FDA's Transition From Computer System Validation To

What is Risk Management? - Steps in Risk Management System

Does FDA Consistently Assess Product Quality Risk and Control in

HIGH-RISK SERIES Efforts Made to Achieve Progress Need to Be

Food and Drug Administration (FDA) Risk Evaluation and Mitigation









