Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization
.png)
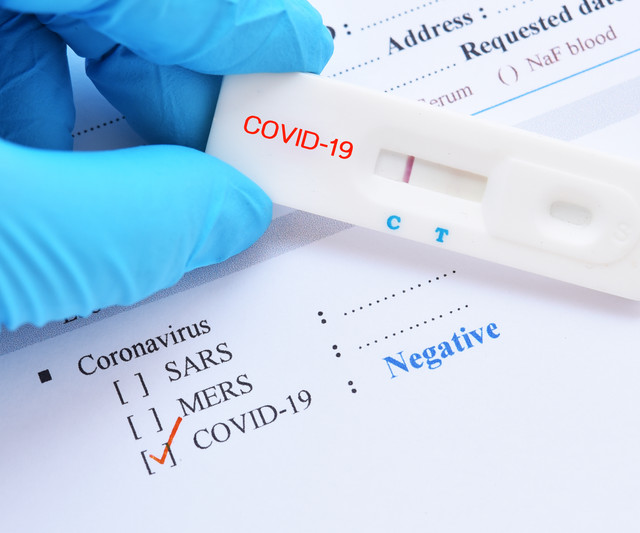
Xpert® Xpress SARS-CoV-2/Flu/RSV received Emergency Use Authorization from the US FDA to support the global fight against COVID-19, with rapid detection of the current coronavirus SARS-CoV-2.

Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization

Cepheid Receives Emergency Use Authorization For SARS-CoV-2, Flu A, Flu B, and RSV Combination Test

Cepheid gets FDA nod for EUA 45-minute coronavirus test, 2020-03-23

Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2 Test - Mar 21, 2020

Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2 Test - COVID-19 - mobile.
.png)
Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization

Cepheid on LinkedIn: Cepheid Receives Emergency Use Authorization from FDA for Rapid SARS-CoV-2…
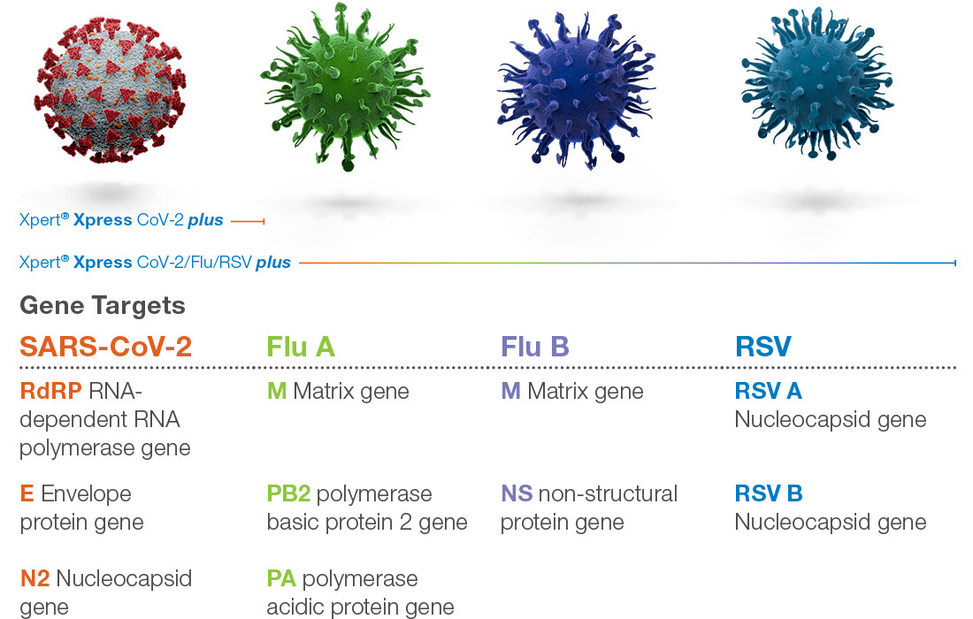
Cepheid Receives Emergency Use Authorization for Xpert® Xpress CoV-2 plus

Cepheid receives EUA from FDA for SARS-CoV-2 Test

Diagnostics, Free Full-Text

Utility of SARS-CoV-2 rapid antigen testing for patient triage in the emergency department: A clinical implementation study in Melbourne, Australia - The Lancet Regional Health – Western Pacific

Design and Implementation of Improved SARS-CoV-2 Diagnostic Assays To Mitigate the Impact of Genomic Mutations on Target Failure: the Xpert Xpress SARS-CoV-2 Experience
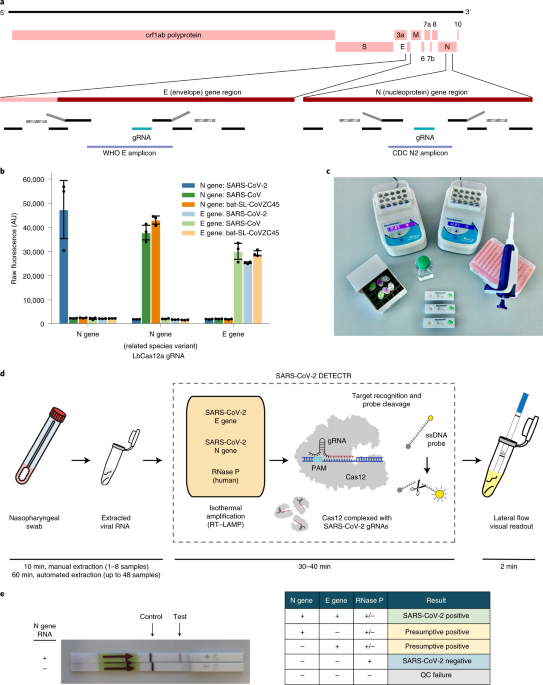
CRISPR–Cas12-based detection of SARS-CoV-2






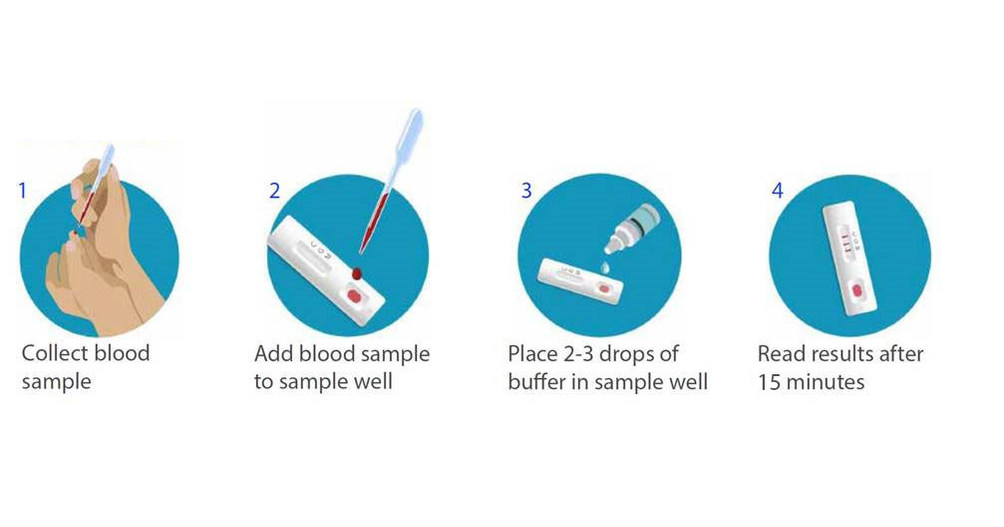
:max_bytes(150000):strip_icc()/combo-rapid-test_04-91f208ea8a2e49dd967da02768978cad.jpg)