Risk Evaluation And Mitigation Strategies (REMS) Basics
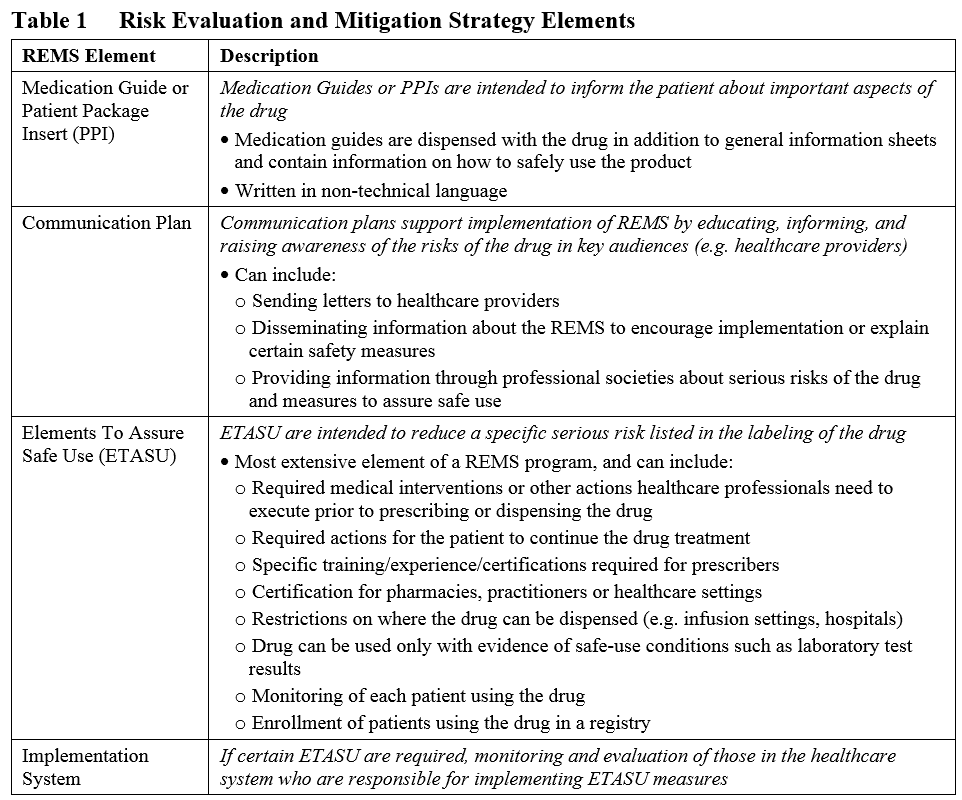

Difference between RMP and REMS – INOPP Forum – VigiServe Foundation

PPT - F OOD AND DRUG ADMINISTRATION AMENDMENTS ACT OF 2007 (FDAAA) and Risk Evaluation and Mitigation Strategies (REMS) PowerPoint Presentation - ID:1328158

What is the Risk Evaluation and Mitigation Strategies (REMS) Program? - GoodRx

Risk Evaluation And Mitigation Strategies (REMS) Basics
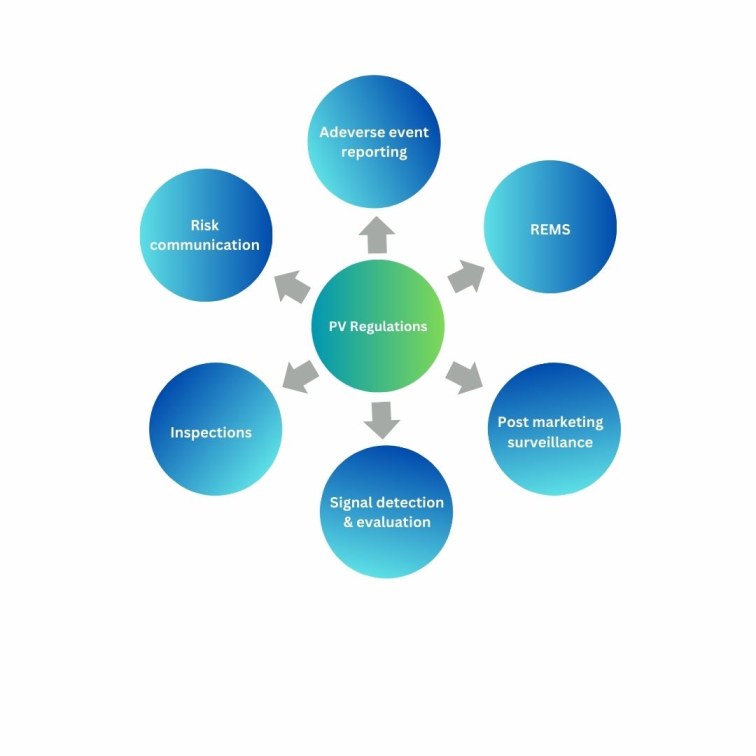
FDA Pharmacovigilance Regulations Update

Roadmap to risk evaluation and mitigation strategies (REMS) success - John D. Balian, Janice C. Wherry, Rachpal Malhotra, Valerie Perentesis, 2010
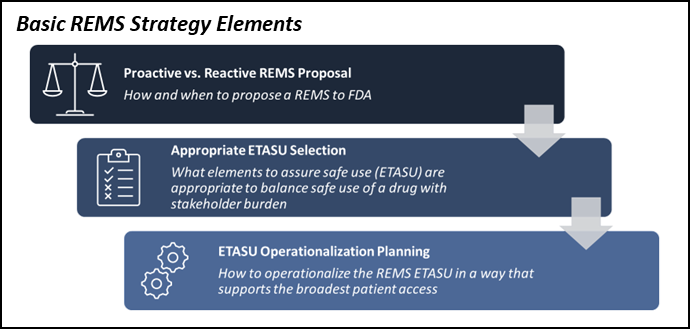
Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy

PPT - F OOD AND DRUG ADMINISTRATION AMENDMENTS ACT OF 2007 (FDAAA) and Risk Evaluation and Mitigation Strategies (REMS) PowerPoint Presentation - ID:1328158
From Our Perspective, A Two-Part Series: Risk Evaluation and Mitigation Strategies (REMS) Program

U.S. Food and Drug Administration - FDA has approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program. Starting on December 13, 2021, patients will be assigned to one

Total number of risk evaluation and mitigation strategy (REMS) programs

REMS in Oncology

Risk management plan








