IQ OQ PQ, Process Validation, Equipment Validation
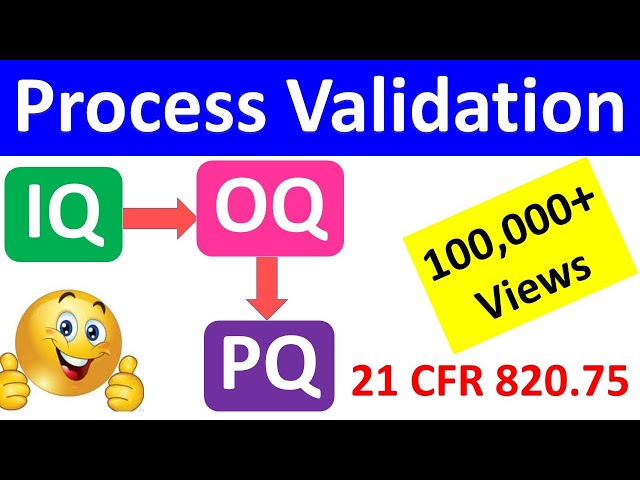
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.
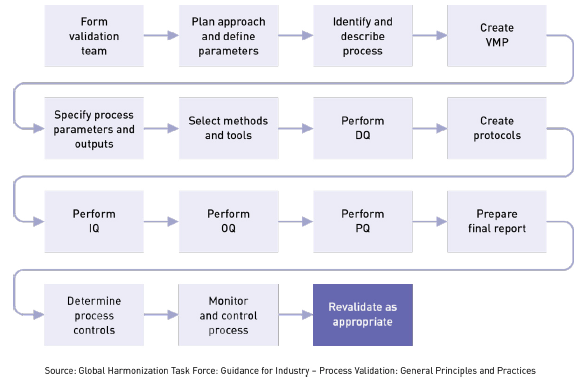
Overview of Medical Device Process Validation: IQ, OQ, and PQ – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
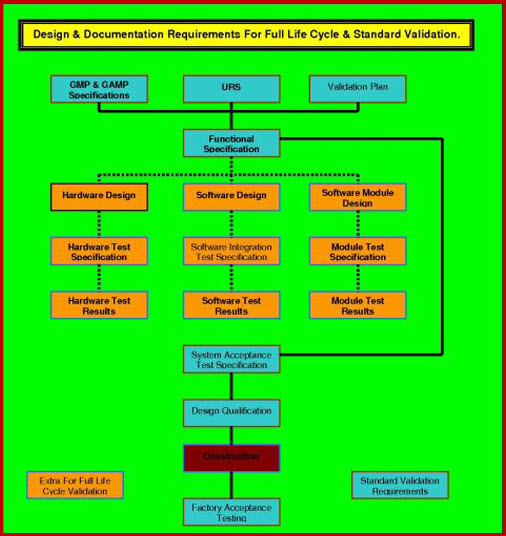
Pharmaceutical Equipment Validation
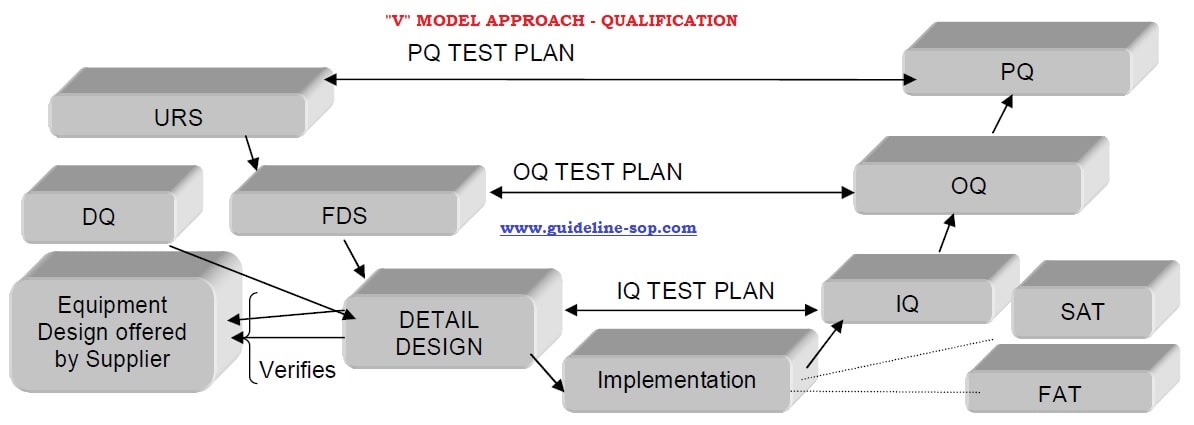
Equipment Qualification Procedure and Protocol - Guidelines - SOPs

Equipment Qualification - IQ, OQ, PQ Protocols : Compliance Training Webinar (Online Seminar)

What Are IQ OQ PQ, The 3 Q's Of Software Validation Process

What Are IQ OQ PQ, The 3 Q's Of Software Validation Process
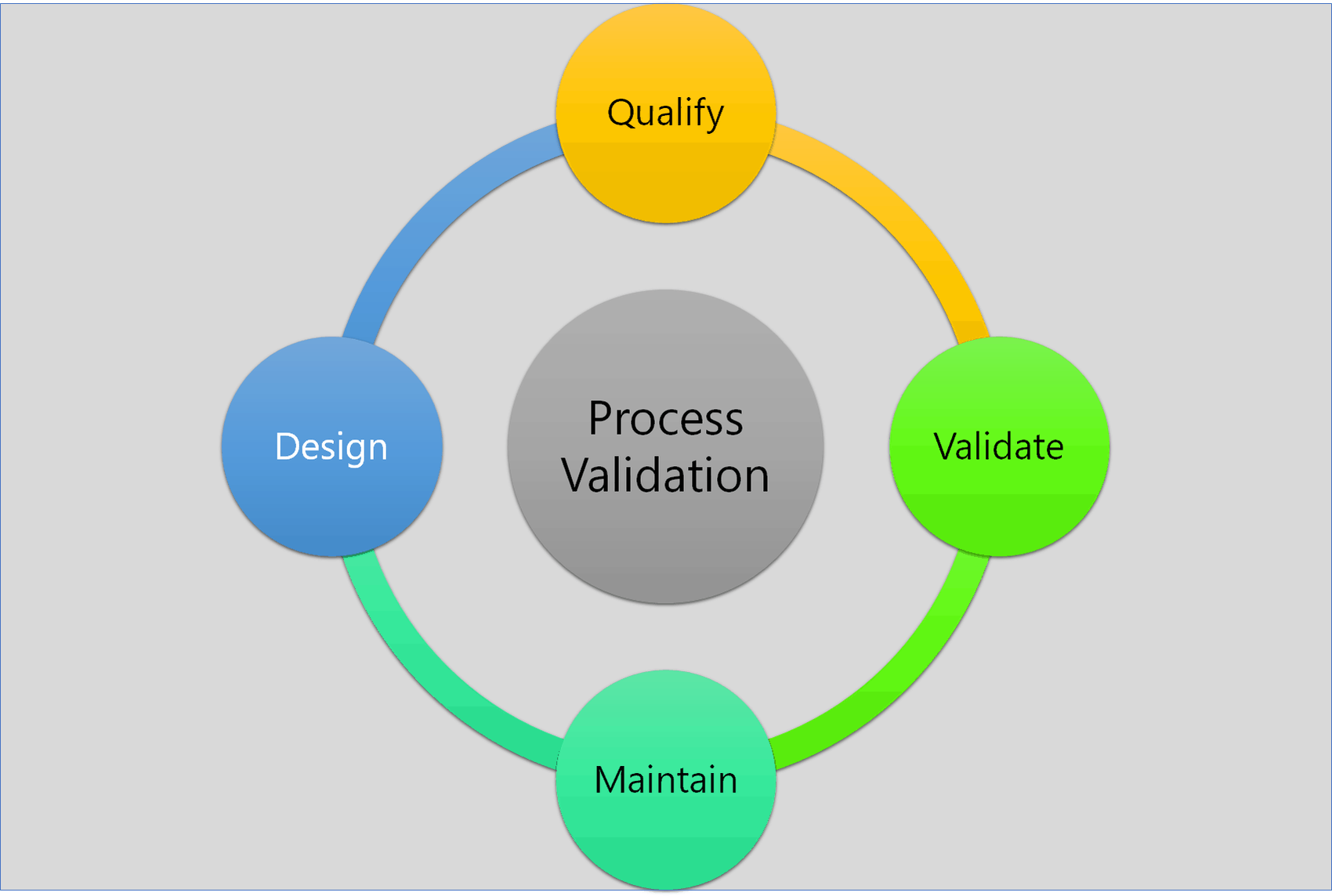
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
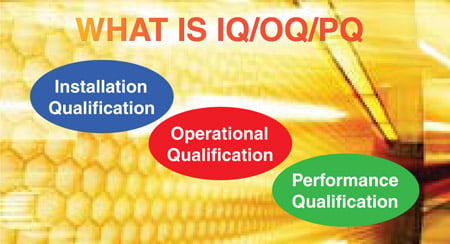
Validation and Qualification - DeltaTrak
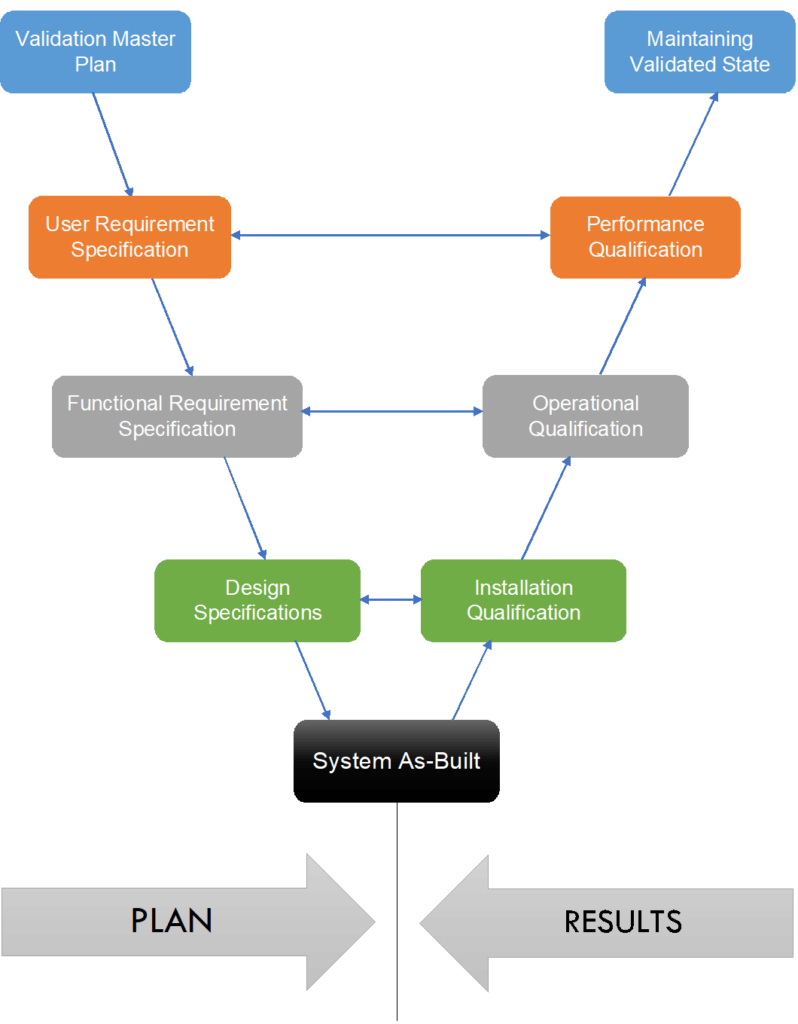
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
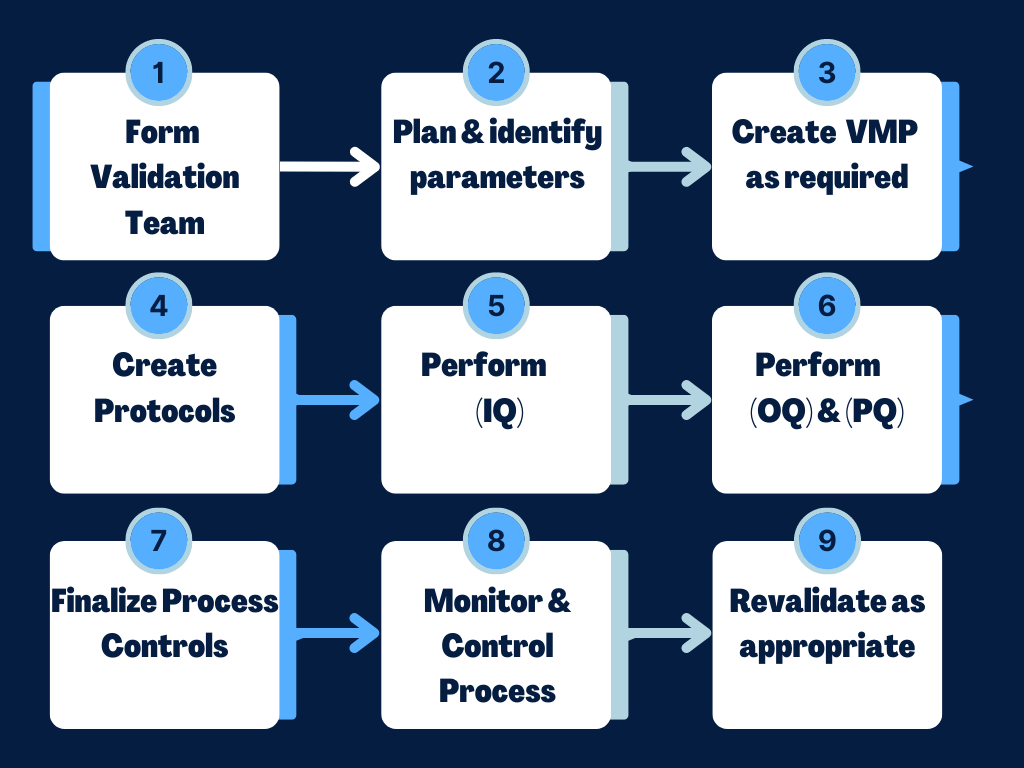
Fast Track ISO 13485 Process Validation Explained for your Medical Device

Equipment Validation : PresentationEZE
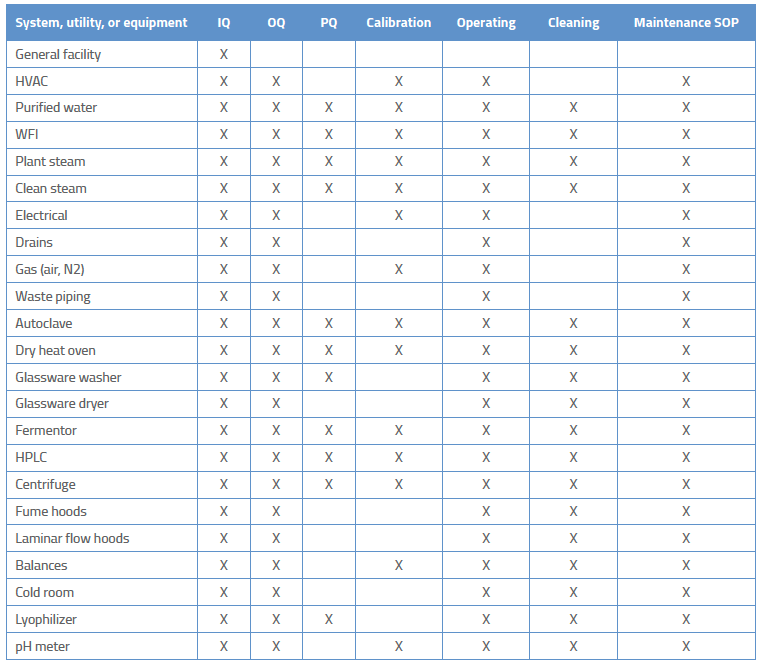
Overview of Medical Device Process Validation: IQ, OQ, and PQ – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

IQ, OQ, PQ - A Validation Process in the Medtech Industry - Elos Medtech