H2O Lewis Structure - Drawing Method of H2O Lewis Structure, Molecular Geometry of H2O, Polarity and Hybridisation in H2O molecule, with FAQs

A Lewis Structure is a simplified representation of the valence shell electrons in a molecule. Water is made up of two hydrogen atoms and one oxygen atom. Dihydrogen monoxide is another chemical name for this molecule.

Give the Lewis structure for H2O (water).
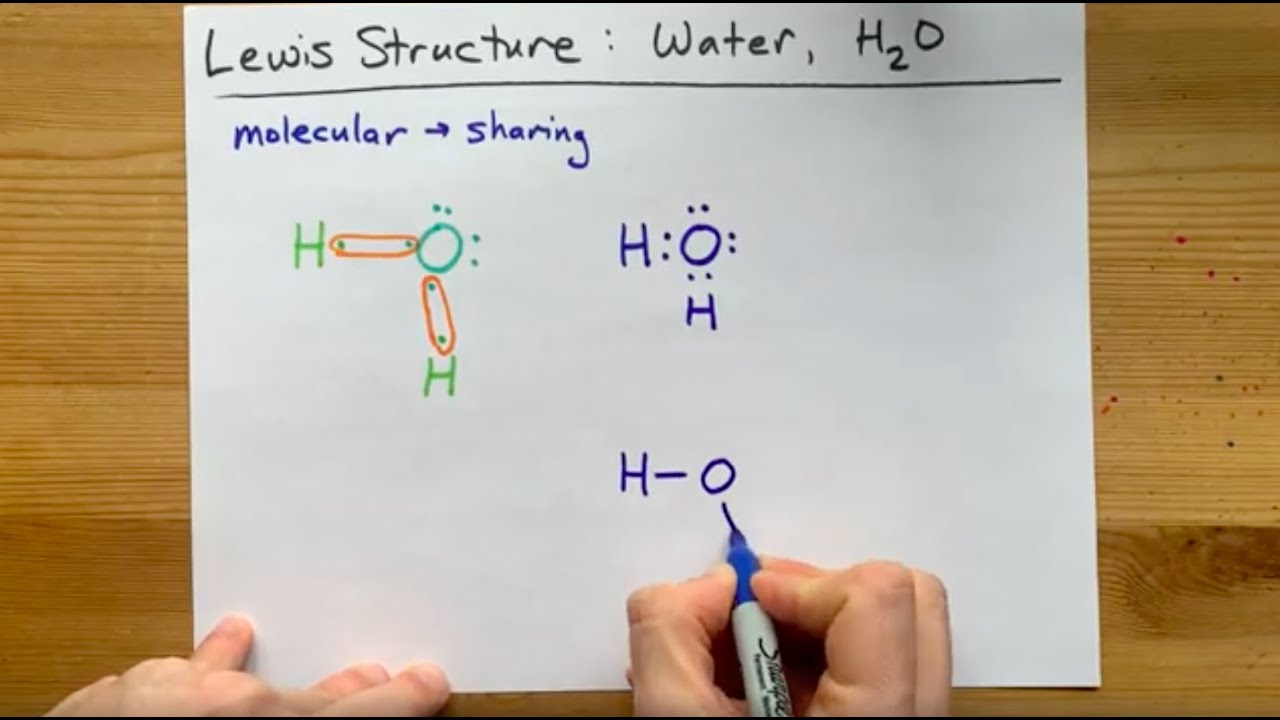
Lewis Structure of H2O, water, dihydrogen monoxide

Water Lewis Structure - How to Draw the Lewis Structure for Water
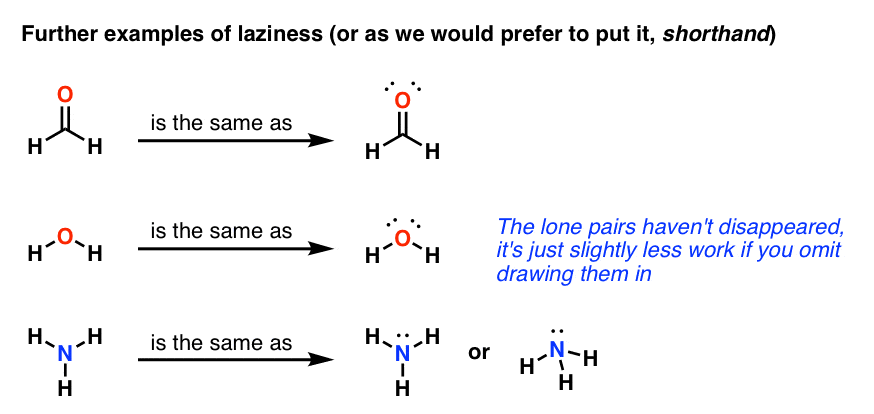
Lewis Structures – Master Organic Chemistry

H2O Lewis Structure (Water)

The Structure and Properties of Water

Draw the Lewis structure for H2O and provide the following information. a. formal charge for each atom b. total number of electron domains c. electron geometry d. molecular geometry e. polarity

Draw the Lewis structure, give the name, and predict the VSEPR geometry of: a) H2O b) NH3 c) SF6

Give the Lewis dot structure of H2O.

Hybridization of H2O (description of hybrid orbitals for O)

Draw the Lewis structure for H2O and state its molecular geometry. Is it polar or nonpolar?