Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is
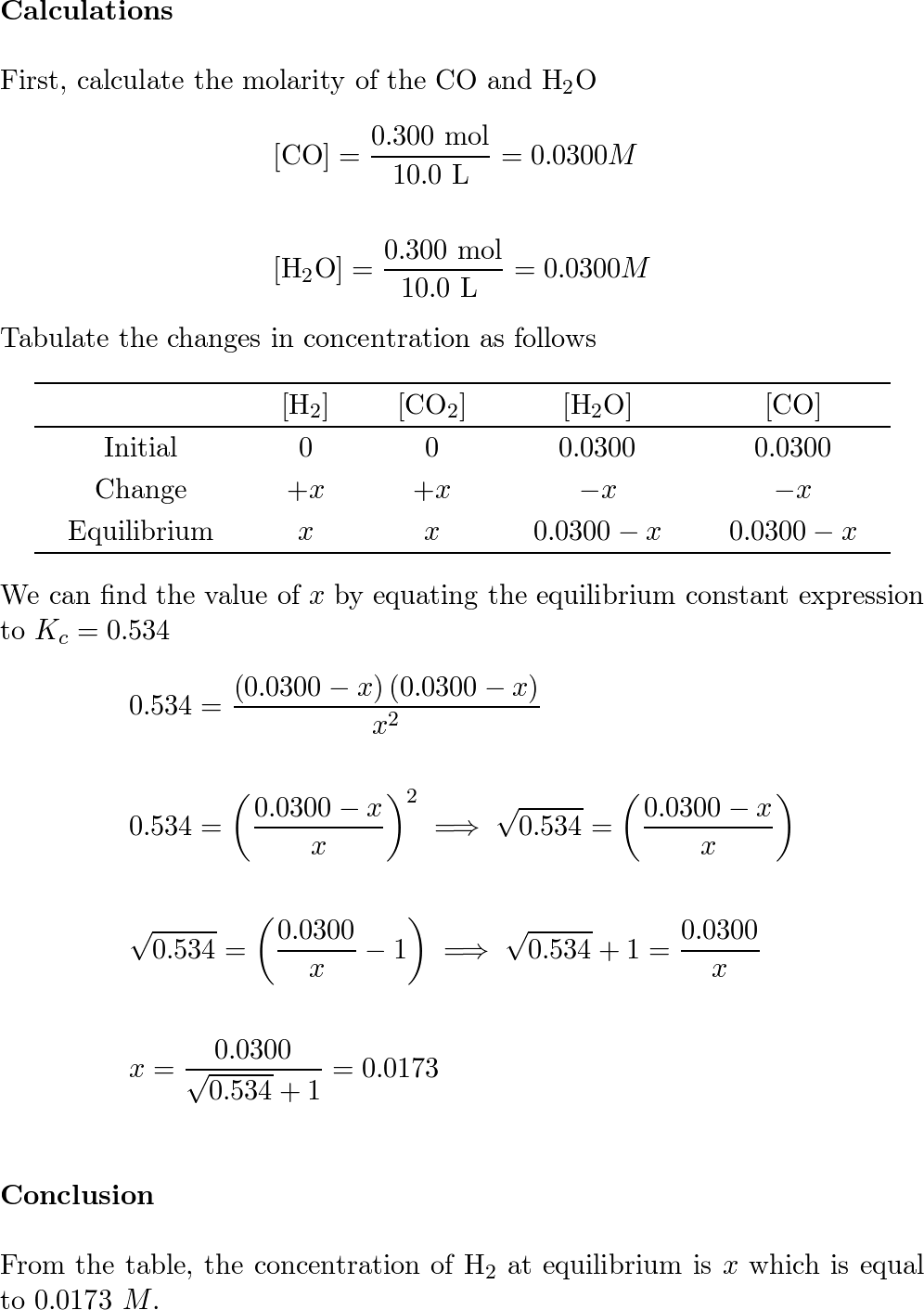
For the reaction H2 (g) + CO2 (g) <=> H2O (g) + CO (g) at 7

Carbon dioxide - Wikipedia

Ch6.1 The Nature of Energy Energy – the capacity to do work or to produce heat. Law of Conservation of Energy – energy can be converted from one form to. - ppt download

20 dm?, AH is - (3) 3.46 kJ (2)-1.73 KJ (1) 1.73 KJ Heat of reaction CO(g) + 12 O. (g) → CO, (g) constant V is -67.71 Kcal 17°C. Thebe constant P 17°C is :- -2.6277 (4) None (3) -67.42 K (2) + 68.0 Kcal (1) -68.0 kcal
Carbon Dioxide, CO2
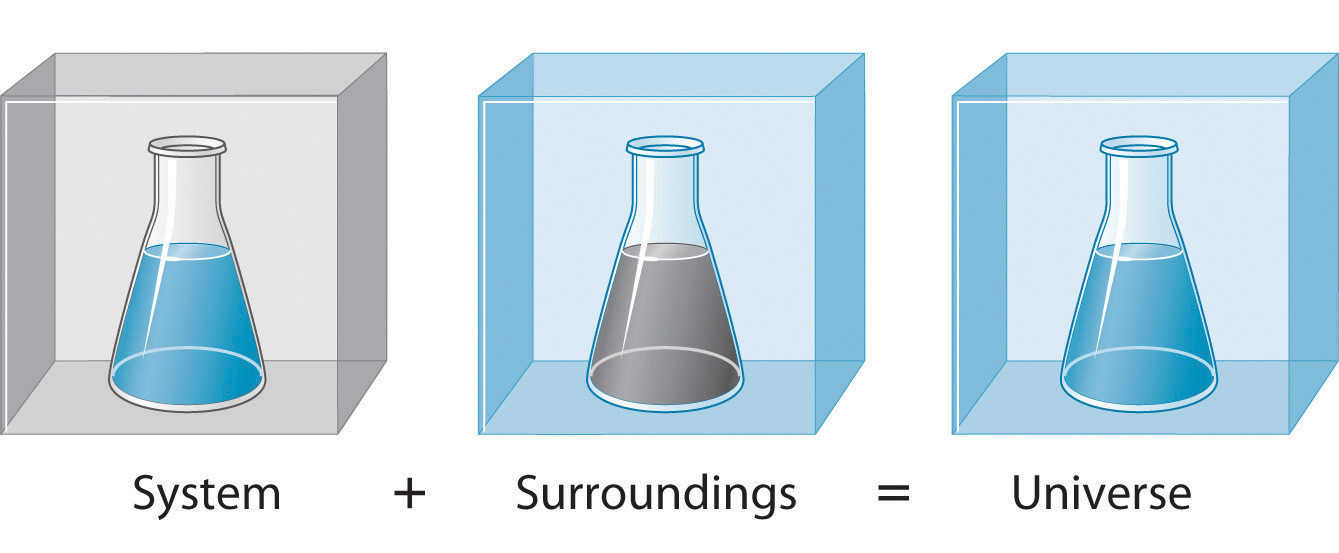
Enthalpy
How to pronounce CH4 + 2O2_ CO2 + 2H2O in terms of mass, particles, moles, volume, and ratio - Quora

PPT - Chapter 14 Heat, Work, Energy, Enthalpy PowerPoint Presentation, free download - ID:4284761
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and H2O(l) are 408, 393, and 286 kj per mol respectively. Calculate heat of combustion of benzoic acid at constant volume
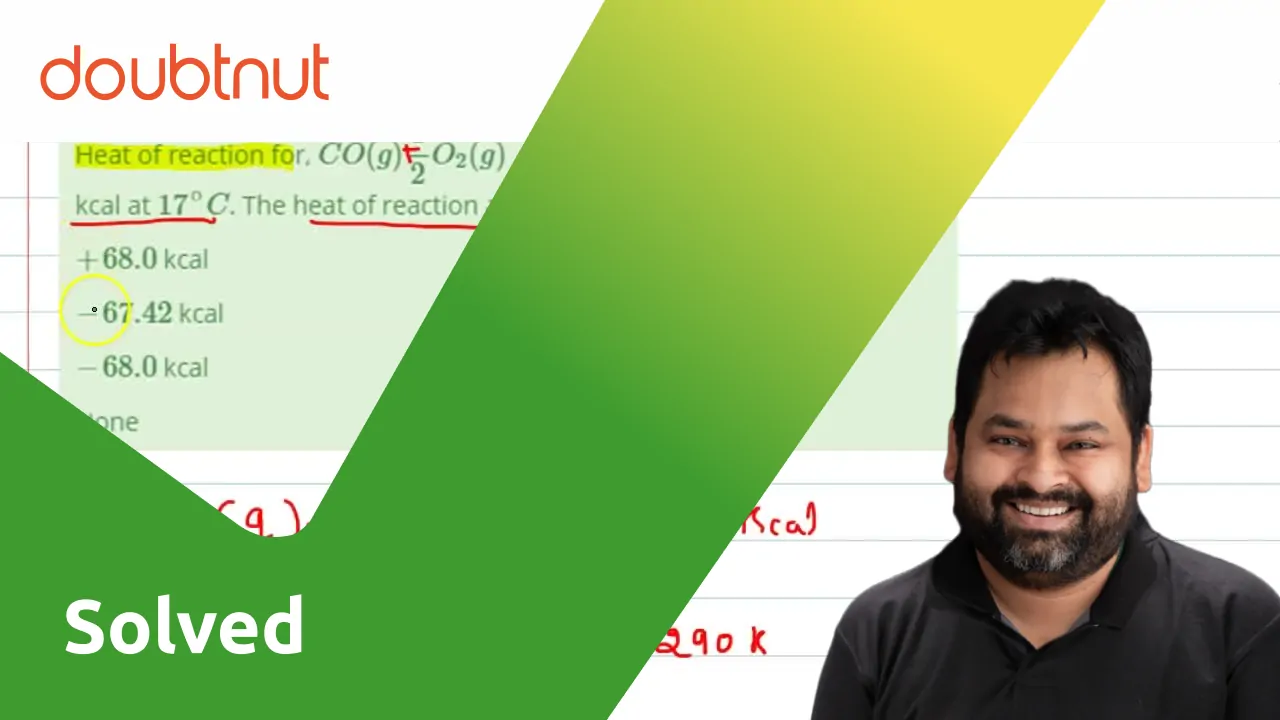
Kannada] Heat of reaction for, CO(g) 1/2 O(2)(g) rarr CO(2)(g) at con